| ќпределение | јлкины Ч это ”¬, в молекулах которых два атома углерода наход€тс€ в состо€нии sp-гибридизации и св€заны друг с другом тройной св€зью (длина св€зи Ч CºCЦ 0,120 нм). |
| ќбща€ формула | CnH2n-2 (n ≥ 2) |
| √омологический р€д, изомери€, номенклатура | 1. Ќ—º—Ќ этин (ацетилен);
радикал Ќ—º—Ц этинил
2. Ќ—º—Ц—Ќ3 пропин (метилацетилен)
3. Ќ—º—Ц—Ќ2ЦCH3 бутин-1(этилацетилен)
CH3ЦCºCЦCH3 бутин-2 (диметилацетилен)
4. HCºCЦCH2ЦCH2ЦCH3 пентин-1
CH3ЦCºCЦCH2ЦCH3 пентин-2
HCºCЦCHЦCH3 3-метилбутин-1
|
—Ќ3
“ипы изомерии:
а) изомери€ цепи;
б) изомери€ положени€ тройной св€зи;
в) межклассова€ изомери€ между алкинами и алкадиенами:
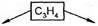 CH3ЦCºCH CH2=C=CH2
пропин пропадиен
CH3ЦCºCH CH2=C=CH2
пропин пропадиен
|
‘изические свойства
—2Ќ2, —3Ќ4, —4Ќ6 —5Ќ8 Е —15Ќ28 C16Ќ30 Е
√азы ∆идкости “вердые вещества
ѕлохо растворимы в воде.
’имические свойства
ƒл€ алкинов, как и дл€ алкенов, характерны реакции присоеди≠нени€. “ак как тройна€ св€зь содержит две π-св€зи, реакции присоединени€ к алкинам могут происходить в две стадии:
X X
| |
(ЦCºCЦ) + X2 Ѓ 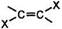 Ѓ ЦCЦCЦ
Ѓ ЦCЦCЦ
| |
X X
ѕрисоединение реагентов типа Ќ’ к несимметричным алкинам происходит по правилу ћарковникова.
| “ип: название реакции | ѕримеры реакций | |
| I. –еакции присоединени€ 1. √идрирование (конечный продукт Ч алканы) | T,Ni HCºCH + H2 Ѓ CH3ЦCH3 этин этан (или постадийно: +H2 +H2 HCºCH Ѓ CH2=CH2 Ѓ CH3ЦCH3) | |
| 2. √алогенирование (конечный продукт Ч тетрагалогеналканы) | Br Br | | HCºCЦCH3 + 2Br2 Ѓ HCЦCЦCH3 (H2O) | | Br Br 1,1,2,2-тетрабромпропан ( ачественна€ реакци€; бромна€ вода обесцве≠чиваетс€) | |
| 3. √идрогалоге-нирование (конеч≠ный продукт Ч дигалогеналканы) | HCºCH + HCl Ѓ CH2=CЦCl ацетилен хлорвинил (ѕолимеризацией хлорвинила получают широкопримен€емый полимер Ч полихлорвинил: T,P,kat n CH2=CHЦCl Ѓ [ЦCH2ЦCHЦ]n) | Cl Cl +HCl +HCl | CH3ЦCºCЦCH3ЃCH3ЦCH=CЦCH3ЃCH3ЦCH2ЦCЦCH3 Cu+,Hg2+ | | Cl Cl бутин-2 2-хлорбутен-2 2,2-дихлорбутан | |
| Br | CH3ЦCºCH + 2HBr Ѓ CH3ЦCЦCH3 | Br пропин 2,2-дибромпропан | ||
| 4. √идратаци€ (образуетс€ ацетальдегид в случае —2Ќ2 и кетоны Ч в случае гомологов ацетилена) Ч реакции учерова | Hg2+
HCºCH + HOH Ѓ [CH2=CH] Ѓ 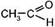 |
OH
этин виниловый спирт ацетальдегид
(неустойчивое соединение)
Hg2+
HCºCЦCH3 + HOH Ѓ CH3ЦCЦCH3
||
O
пропин диметилкетон(пропанон, ацетон)
|
OH
этин виниловый спирт ацетальдегид
(неустойчивое соединение)
Hg2+
HCºCЦCH3 + HOH Ѓ CH3ЦCЦCH3
||
O
пропин диметилкетон(пропанон, ацетон)
| |
| II. –еакции окис- лени€ 1. √орение | T 2C2H2 + 5O2 Ѓ 4CO2 + 2H2O (горит копт€щим пламенем) | |
| 2. Ќеполное окисление под действием ћnќ4 (образуютс€ карбоновые кисло- ты) |
HºCºCH + 4(O) Ѓ 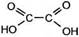 Ётин (из ћnќ4) этандиова€
(щавелева€) кислота
HCºCЦCH3 + 3(O) + H2O Ѓ
Ётин (из ћnќ4) этандиова€
(щавелева€) кислота
HCºCЦCH3 + 3(O) + H2O Ѓ 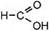 + + 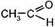 пропин метанова€ этанова€
(муравьина€) (уксусна€)
кислота кислота
CH3ЦCºCЦCH3 + 3(O) + H2O Ѓ 2
пропин метанова€ этанова€
(муравьина€) (уксусна€)
кислота кислота
CH3ЦCºCЦCH3 + 3(O) + H2O Ѓ 2 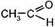 бутин-2
( ачественные реакции; р-р ћnќ4 обесцвечиваетс€)
бутин-2
( ачественные реакции; р-р ћnќ4 обесцвечиваетс€)
| |
| III. –еакции ди-, три- и полимеризации | CuCl2,H+ HCºCH + HCºCH Ѓ CH2=CHЦCºCH ацетилен винилацетилен (димер) »з винилацетилена присоединением HCI получают хлоропрен, при полимеризации которого образуетс€ хлоропреновый каучук: Cl | CH2=CHЦCºCH + HCl Ѓ CH2=CHЦC=CH2 Cl Cl | T,P,kat | nCH2=CHЦC=CH2 Ѓ (ЦCH2ЦCH=CЦCH2Ц)n | |
| ÷иклотримеризаци€ | C(act),~5000C
3HCºCH Ѓ 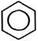 ацетилен бензол
H2SO4,T
3CH3ЦCºCH Ѓ
ацетилен бензол
H2SO4,T
3CH3ЦCºCH Ѓ 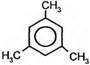 1,3,5-триметилбензол
ѕолимеризаци€ дл€ алкинов малохарактерна
1,3,5-триметилбензол
ѕолимеризаци€ дл€ алкинов малохарактерна
| |
| IV. –еакции замещеи€ (образуютс€ нерастворимые соли Чацетилениды). ¬озможны только дл€ акинов, содер≠жащих этинильную группу ЦCºCH (т. н. терминальные алкины) | HCºCH + 2CuCl Ѓ CuЦCºCЦCu¯ + 2HCl Ётин ацетиленид меди (I) двузамещенный CH3ЦCºCH + CuCl Ѓ CH3ЦCºCЦCu¯+ HCl пропин метилацетиленид меди (I) (ќбразование темно-красных осадков ацетиленидов меди Ч качественна€ реакци€ на этинильную группу, позвол€юща€ отличить терминальные алкины от других непредельных ”¬) NH3(жид.) 2HCºCH + 2Na Ѓ 2HCºCЦNa + H2≠ ацетиленид натри€ однозамещенный NH3,Ќ2ќ HCºCH + A2O Ѓ AgЦCºCЦAg¯ + H2O ацетиленид серебра двузамещенный |
|
|
|
—пособы получени€
| Ќазвание способа | ”равнени€ реакций |
| 1. арбидный способ | јцетилен CaC2 + 2H2O Ѓ HCºCH≠ + Ca(OH)2 карбид кальци€ |
| 2. “ермическое разложение природного газа (метана) | ~12000C 2CH4 Ѓ HCºCH + 3H2 метан |
| 3. ƒегидрогалогенирование дигалогеналканов при действии избытка спирто≠вого раствора щелочи | ќбщие дл€ —2Ќ2 и его гомологов »з дигалогеналканов, содержащих атомы га≠логена у двух соседних атомов углерода, напри≠мер: T CH3ЦCHЦCHЦCH3+2KOHЃCH3ЦCºCЦCH3+2KCl+ 2H2O | | Cl Cl 2,3-дихлорбутан бутин-2 »з дигалогеналканов, содержащих два атома галогена у одного атома углерода, например: T CH3ЦCH2ЦCHBr2+2NaOHЃCH3ЦCºCH +2NaBr+2H2O 1,1-дибромпропан пропин |






