в) нитрата натри€ (NaNO3)?
–≈Ў≈Ќ»≈
 Al Cl3 + HOH <=> AlOHCl2 + HCl
Al Cl3 + HOH <=> AlOHCl2 + HCl
 Al (OH)3 + HCl
Al (OH)3 + HCl
|
|

 Al3+ + H+ OH - <=> +
Al3+ + H+ OH - <=> +
 |
ак видно из сокращенного ионно-молекул€рного уравнени€, при гидролизе образуютс€ катионы Ќ+, обуславливающие кислую среду раствора (рЌ<7).
–еакци€ гидролиза Ц процесс обратимый, поэтому равновесие гидролиза соли может смещатьс€ при введении в раствор некоторых веществ (согласно правилу смещени€ равновеси€ по принципу Ће Ўателье).
а) ≈сли к раствору AlCl3 добавить бромоводородную кислоту HBr, то в результате еЄ диссоциации (HBr = H+ + Br -) в растворе увеличитс€ концентраци€ ионов H+, вследствие чего равновесие реакции гидролиза AlBr3 сместитс€ в левую сторону (образование исходных веществ), т.е. гидролиз AlBr3 ослабевает.
|
 ←
←
Al3+ + HOH <=> AlOH2+ +
HBr = Br - +
б) ≈сли же к раствору AlCl3 добавить щелочь ( ќЌ), то в результате ее диссоциации ( ќЌ = + + ќЌ-) в растворе по€вл€ютс€ ионы ќЌ-, которые с ионами Ќ+, полученными в реакции гидролиза, образуют молекулу слабого электролита (H2O), что приведет к уменьшению концентрации ионов Ќ+ и смещению равновеси€ реакции гидролиза в правую сторону, т.е. гидролиз AlCl3 усилитс€.
|
 →
→
Al3+ + HOH <=> AlOH2+ + → Ќ2ќ
ќЌ = + +
в) Ќитрат натри€ Ц сильный электролит, который не имеет общих ионов ни с AlCl3, ни с продуктами его гидролиза, и не св€зывает их в слабодиссоциирующие соединени€, т.е. прибавление NaNO3 на равновесие гидролиза не вли€ет.
 Al3+ + HOH <=> AlOH2+ + H+
Al3+ + HOH <=> AlOH2+ + H+
NaNO3 = Na+ + NO3-
3. ”казать, кака€ из двух солей хлорид цинка (ZnCl2) или хлорид меди (—uCl2), при равных концентраци€х и температурах в большей степени подвергаетс€ гидролизу? ќтвет мотивировать сравнением значений констант диссоциации слабых электролитов, образующих обе соли, по последней ступени. —оставить ионно-молекул€рные и молекул€рные уравнени€ гидролиза этих солей.
ƒано:
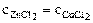 “ = const
“ = const
| –≈Ў≈Ќ»≈: |
| ”казать, как€ соль (ZnCl2 или CuCl2 ) в большей степени подвергаетс€ гидролизу. |

 Zn Cl2 + H2O <=> ZnOHCl + HCl
Zn Cl2 + H2O <=> ZnOHCl + HCl
Zn (OH)2 + HCl
|
|

 Zn 2+ + H OH < = > +, рЌ < 7 - среда
Zn 2+ + H OH < = > +, рЌ < 7 - среда
 кисла€
кисла€
Cu Cl2 + H2O < = > CuOHCl + HCl

 Cu (OH)2 + HCl
Cu (OH)2 + HCl
|
|
Cu2+ + H OH < = > +, рЌ < 7 - среда


 кисла€
кисла€
 (табл.).
(табл.).
 (табл.)
(табл.)
“.к.  <
<  , то Cu(OH)2 Ц более слабый электролит, чем Zn(OH)2. ѕоскольку степень гидролиза соли h тем больше, чем слабее электролит, образующий эту соль (h ~ 1/K), CuCl2 в большей степени подвергаетс€ гидролизу.
, то Cu(OH)2 Ц более слабый электролит, чем Zn(OH)2. ѕоскольку степень гидролиза соли h тем больше, чем слабее электролит, образующий эту соль (h ~ 1/K), CuCl2 в большей степени подвергаетс€ гидролизу.
ќтвет: ѕри сравнении значений констант диссоциации по последней ступени слабых электролитов, образующих соли ZnCl2 и CuCl2,  <
<  , поэтому CuCl2 в большей степени подвергаетс€ гидролизу, чем ZnCl2.
, поэтому CuCl2 в большей степени подвергаетс€ гидролизу, чем ZnCl2.
|
|
|
”–ќ¬≈Ќ№ ¬
1. —оставить ионно-молекул€рное и молекул€рное уравнени€ гидролиза карбоната кали€ (K2CO3). ¬ычислить константу, степень и рЌ гидролиза соли в 0,01ћ растворе.
| –≈Ў≈Ќ»≈: |
 |
K2 CO3 + H2O <=> KHCO3 + KOH

 KOH + H2 CO3
KOH + H2 CO3
|
|

 —ќ32 - + Ќ ќЌ <=> +, рЌ>7, среда
—ќ32 - + Ќ ќЌ <=> +, рЌ>7, среда
 щелочна€
щелочна€
Kг = 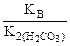 ,
,
 (табл.).
(табл.).
Kг =  ,
,
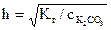 ,
,  =0,14,
=0,14,
pH+ рќЌ=14.
“ак как среда щелочна€, то определ€ем  :
:
pќЌ= -1/2∙lgKг -1/2∙lg  =
=
=  2,84
2,84
рЌ = 14-рќЌ, рЌ = 14-2,84=11,16,
ќтвет: 
2. –ассчитать при температуре 300 константу, степень и рЌ гидролиза нитрат аммони€ (NH4NO3) в 1ћ растворе, использу€ значени€ термодинамических характеристик реакции гидролиза соли. Ќаписать ионно-молекул€рное и молекул€рное уравнение гидролиза этой соли.
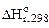 = 51,14 кƒж;
= 51,14 кƒж;
 = - 4,67 ƒж/ .
= - 4,67 ƒж/ .
| –≈Ў≈Ќ»≈: |
NH4 NO3 + H2O <=> NH4OH + HNO3

 NH 4OH + HNO3
NH 4OH + HNO3
|
NH4 + + H OH <=> NH4OH +, pH<7, среда


 кисла€
кисла€
 (8.1)
(8.1)
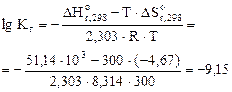
 ,
,
 =2,67·10-5
=2,67·10-5
“.к. среда кисла€, то определ€ем рЌ:



 ,
,
рЌ = -1/2∙(-9,15)-1/2∙lg1= 4,60.
ќтвет: 
3. „то произойдет при сливании растворов хлорида железа (II) (FeCl2) и карбоната натри€ (Na2CO3)? Ќаписать уравнени€ реакций в ионно-молекул€рной и молекул€рной формах.

| –≈Ў≈Ќ»≈:
ƒо сливани€ в растворе каждой соли протекает ее гидролиз по I ступени (гидролизу подвергаютс€ ионы Fe2+ и CO  ):
Fe Cl2
Fe (OH)2 + HCl слаб. сильн.
Na2 CO3
NaOH + H2 CO 3
сильн. слаб. ):
Fe Cl2
Fe (OH)2 + HCl слаб. сильн.
Na2 CO3
NaOH + H2 CO 3
сильн. слаб.
|
|
Fe2+ +HOH <=> FeOH+ + → Ќ2ќ
CO32-+ HOH <=> HCO3-+
ѕосле сливани€ растворов продукт гидролиза первой соли (Ќ+) взаимодействует с продукт  ом гидролиза второй соли (ќЌ-) с образованием слабодиссоциирующего соединени€ Ќ2ќ, что приводит к смещению химического равновеси€ в сторону пр€мых реакций. ”силение гидролиза первой и второй соли приводит к протеканию второй ступени гидролиза с образованием осадка и выделением газа, т.е. гидролиз протекает необратимо, до конца.
ом гидролиза второй соли (ќЌ-) с образованием слабодиссоциирующего соединени€ Ќ2ќ, что приводит к смещению химического равновеси€ в сторону пр€мых реакций. ”силение гидролиза первой и второй соли приводит к протеканию второй ступени гидролиза с образованием осадка и выделением газа, т.е. гидролиз протекает необратимо, до конца.
|
FeOH+ + HOH = Fe(OH)2↓ + + → Ќ2ќ
HCO3- + HOH = H2CO3 +


CO2↑ H2O
—окращенное ионно-молекул€рное уравнение гидролиза двух солей:
Fe2+ + CO32- + H2O = Fe(OH)2↓ + CO2↑.
ћолекул€рное уравнение совместного гидролиза:
FeCl2 + Na2CO3 + H2O = Fe(OH)2↓ + CO2↑ + 2NaCl





 Kг -? h -? pH-?
Kг -? h -? pH-?
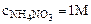 T=300K
Kг-? h-? pH-?
T=300K
Kг-? h-? pH-?


