ѕример 20 поможет ¬ам при решении задач є 142,144Ц146.
ѕример 20. ѕостроить кривую титровани€ NaOH [—(NaOH) = = 0,15 моль/л] раствором HCl c концентрацией 0,30 моль/л и подобрать индикаторы.
–ешение. NaOH Ц сильное основание, а HCl Ц сильна€ кислота, поэтому в данном случае рассмотрим титрование сильного основани€ сильной кислотой.
ѕредположим, что дл€ титровани€ вз€ли 100,00 мл (V0) раствора NaOH [—0 = —(NaOH) = 0,15 моль/л]. ѕо закону эквивалентов рассчитаем объем раствора HCl (V х), необходимый дл€ оттитровывани€ NaOH (дл€ достижени€ т. э):
100,00 Ј 0,15 = V х Ј 0,30; V х = 50,00 мл.
ƒл€ простоты расчета допустим, что объем раствора при титровании не измен€етс€.
1. —ильные кислоты и основани€ в водном растворе диссоциированы нацело, поэтому концентрацию Ќ+ или рЌ рассчитывают, исход€ из концентрации NaOH по уравнению (3).
pH = 14 Ц pOH = 14+lg 0,15 = 13,18.
2. «начени€ рЌ раствора до точки эквивалентности будут определ€тьс€ концентрацией неоттитрованного основани€. ѕри прибавлении 25,00 мл (50%) раствора HCl на титрование затрачено n(HCl) = = 25,00 Ј 10Ц3 Ј 0,30 = 7,50 Ј 10Ц3 моль. Ќеоттитрованное количество NaOH определ€етс€ вычитанием эквивалентного количества от исходного:
100,00 Ј 10Ц3 Ј 0,15 Ц 7,50 Ј 10Ц3 = 7,50 Ј 10Ц3 моль.
ќтсюда —(NaOH) = 7,50 ∙ 10Ц3/100,00 ∙ 10Ц3 = 7,50 ∙ 10Ц2 моль/л, а
рЌ = 14 + lg 7,50 ∙ 10Ц2 = 12,88.
ќбъедин€€ поэтапный расчет и учитыва€, что мол€рные концентрации эквивалентов в данном случае равны мол€рным концентраци€м, получим в общем виде:
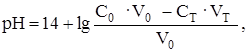
где —т и —0 Ц исходные концентрации растворов титранта и титруемого вещества соответственно; V0 Ц исходный объем титруемого вещества; Vт Ц добавленный объем титранта.
“аким же образом рассчитываем рЌ при прибавлении 45,00 мл раствора HCl (45,00: 50,00 Ј 100 = 90%):
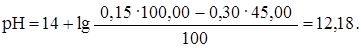
≈сли добавлено 49,50 мл раствора HCl (49,50: 50,00 Ј 100 = = 99%), то
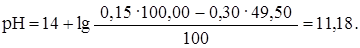
ѕосле прибавлени€ 49,95 мл раствора HCl (49,95: 50,00 Ј 100 = = 99,9%):

3. ¬ точке эквивалентности рЌ раствора определ€етс€ автопротолизом воды, рЌ = 1/2 р W = 7.
«а точкой эквивалентности рЌ определ€етс€ избытком ЌCl. ѕри добавлении 50,05 мл раствора HCl (50,05: 50,00 Ј 100 = 100,1 %):

а так как в точке эквивалентности количество моль титруемого вещества равно количеству моль титранта, то есть —0 ∙ V0= 100,00 Ј 0,15 = = 50,00 ∙ 0,30, то:

ѕри добавлении 50,50 мл раствора HCl (50,50: 50,00 Ј 100 = = 101 %):
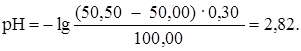
ѕри добавлении 55,00 мл раствора HCl (55,00: 50,00 Ј 100 = = 110 %):
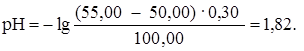
–езультаты вычислений сведем в табл. 1, а по данным таблицы построим кривую титровани€ (рис. 1).
“аблица 1
| ќбъем титранта VT, мл | рЌ-определ€ющий компонент | ‘ормула дл€ расчета рЌ | рЌ |
| NaOH | 14+lg —0(NaOH) | 13,18 | |
| 25,00 | NaOH | 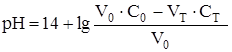
| 12,88 |
| 45,00 | NaOH | -″- | 12,18 |
| 49,50 | NaOH | -″- | 11,18 |
| 49,95 | NaOH | -″- | 10,18 |
| 50,00 | H2O | рЌ = 1/2 р W | 7,00 |
| 50,05 | HCl | 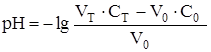
| 3,82 |
| 50,50 | HCl | -″- | 2,82 |
| 55,00 | HCl | -″- | 1,82 |
—качок титровани€ определ€етс€ резким изменением рЌ от 10,18 до 3,82 при недотитровывании или перетитровывании на 0,1% соответственно. ѕравило выбора индикаторов предусматривает, что интервал перехода окраски индикатора должен укладыватьс€ в скачок титровани€. »спользу€ справочные данные по интервалу перехода окраски индикаторов, предлагаем следующие наиболее часто примен€емые индикаторы: метиловый оранжевый (интервал перехода 3,1Ц4,4; р“=4,0), метиловый красный (интервал перехода 4,2Ц6,2; р“=5,0), бромтимоловый синий (интервал перехода 6,0Ц7,6; р“=7,0), фенолфталеин (интервал перехода 8,2Ц10,0; р“=9,0).
|
|
|

–ис. 1. рива€ титровани€ 0,15 моль/л раствора гидроксида натри€ 0,30 моль/л раствором сол€ной кислоты






