ƒл€ проведени€ расчетов в титриметрии пользуютс€ пон€тием эквивалента и фактора эквивалентности. Ёквивалентом называют условную частицу вещества, равноценную в данной реакции одному иону водорода или одному электрону. ‘актор эквивалентности (fэкв., 1/ Z) определ€ет долю реальной частицы вещества, по которой рассчитываетс€ эквивалент. ¬ расчетах, св€занных с определением фактора эквивалентности и мол€рной массы эквивалента, прежде всего необходимо учитывать тип протекающей реакции.
1. ¬ кислотно-основной реакции эквивалент кислоты или основани€ Ц така€ условна€ частица вещества, котора€ в данной реакции высвобождает один ион водорода или соедин€етс€ с ним, или каким-либо образом эквивалентна ему. Ќапример, рассчитаем fэкв.(CaCO3) в реакции
CaCO3 + 2H+ + 2ClЦ = H2CO3 + Ca2+ + 2ClЦ
ќтсюда fэкв.(CaCO3) = 1/ Z = 1/2, а мол€рна€ масса эквивалента = = ћ(1/2 CaCO3) = ћ(CaCO3) ∙ 1/2.
2. ¬ реакции окислени€-восстановлени€ эквивалент окисл€ющегос€ или восстанавливающегос€ вещества Ц это така€ условна€ частица вещества, котора€ может присоедин€ть или отдавать один электрон в данной химической реакции. ƒл€ примера определим fэкв.(Na2S2O3) и продукта его окислени€ Ц иона 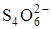 , использу€ полуреакцию
, использу€ полуреакцию
2 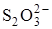 Ц 2ē D
Ц 2ē D 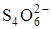 ,
,
в которой участвуют два электрона. ќдин ион 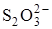 отдает 1 электрон, то есть fэкв.(S2O32Ц) = fэкв.(Na2S2O3) = 1, а один ион
отдает 1 электрон, то есть fэкв.(S2O32Ц) = fэкв.(Na2S2O3) = 1, а один ион 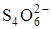 присоедин€ет 2 электрона (z =2), и fэкв.(
присоедин€ет 2 электрона (z =2), и fэкв.(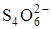 ) = fэкв.(Na2S4O6) = 1/ z = = 1/2 .
) = fэкв.(Na2S4O6) = 1/ z = = 1/2 .
3. ¬ комплексонометрии комплексон III (синонимы Ц Ёƒ“ј, трилон Ѕ), представл€ющий собой двунатриевую соль этилендиаминтетрауксусной кислоты Ц Na2H2Y, в водном растворе диссоциирует с образованием ионов H2Y2Ц. ƒл€ определени€ fэкв.(комплексона III) и fэкв.(ћеn+) используем реакцию
H2Y2Ц + ћеn+ D ћеY2Ц + 2 Ќ+.
¬ результате замещени€ выдел€етс€ 2Ќ+, отсюда следует, что fэкв.(комплексона III) равен 1/2. ќсобенностью реакции €вл€етс€ то, что при взаимодействии H2Y2Ц с ионами металлов в разной степени окислени€ (n>2) всегда освобождаютс€ только 2 иона водорода (z =2), и в этом случае fэкв.(комплексона III) = fэкв.(ћеn+)= 1/2. Ќапример, при титровании иона Al3+ комплексоном III:
H2Y2Ц + Al3+ D AlYЦ + 2 Ќ+, и fэкв.(Al3+) = 1/2.
4. ¬ случае, если одновременно используютс€ реакции различных типов, например осаждени€ и окислени€-восстановлени€, комплексообразовани€ и окислени€-восстановлени€ и т. д., фактор эквивалентности определ€етс€ с помощью пропорции. –асчет фактора эквивалентности показан в примере 18.
—пособы титровани€
–асчеты результатов анализа завис€т от способов титровани€. –азличают методы пр€мого титровани€, обратного и по заместителю.
¬ методе пр€мого титровани€ к некоторому объему анализируемого вещества (V х) непосредственно добавл€ют титрант с известной мол€рной концентрацией эквивалента (—“). ќпределив объем титранта (V“) в т. э., рассчитывают мол€рную концентрацию эквивалента (— х) или количество молей эквивалента анализируемого вещества (n х) по закону эквивалентов:
— х ∙ V х = —“ ∙ V“;
|
|
|
n х = n“;
n х = —“ ∙ V“.
ћассу определ€емого вещества (m x) рассчитывают по формуле
m x = n х ∙ M(1/ ZX) ∙ c“ ∙ V“ ∙ M(1/ ZX),
где M(1/ Z X) Ц мол€рна€ масса эквивалента определ€емого вещества.
ћетод обратного титровани€ заключаетс€ в прибавлении к раствору анализируемого вещества избытка точно известного количества стандартного раствора ¬ и после завершени€ реакции между ними титровании оставшеес€ количество вещества ¬ раствором титранта —. оличество молей эквивалента определ€емого вещества равно разности между количеством молей эквивалента веществ ¬ и —:
n х = n¬ Ц n—.
¬ методе титровани€ по заместителю к определ€емому веществу добавл€ют реагент, вступающий с ним в реакцию. «атем один из продуктов взаимодействи€ (¬) оттитровывают титрантом —. оличество молей эквивалента определ€емого вещества при титровании заместител€ всегда равно количеству молей эквивалента титранта:
n х = n¬ = n—.
–ј—„≈“џ –≈«”Ћ№“ј“ќ¬
“»“–»ћ≈“–»„≈— ќ√ќ јЌјЋ»«ј






