Ќаличие, хоть и очень небольших, концентраций ионов, образующихс€ при диссоциации растворител€, приводит к их взаимодействию с ионами растворенных в данном растворителе веществ и возникновению новых ионных равновесий. Ёто €вление в общем случае называетс€ сольволиз, а если растворитель Ч вода, то гидролиз. ƒопустим, что мы растворили в воде соль слабой кислоты и сильного основани€, например, ацетат натри€. “огда получим два равновеси€:
CH3COONa = —Ќ3—ќOЦ + Na+
2H2O = H3O+ +OHЦ
јцетат натри€ в воде €вл€етс€ сильным электролитом, т. е. распадаетс€ на ионы практически нацело. ћежду ионами в растворе возникает взаимодействие
Na+ + ќЌЦ = NaOH
—Ќз—ќOЦ + Ќ3ќ+ = —Ќз—ќќЌ + Ќ2ќ
Ќо щелочь €вл€етс€ также сильным электролитом, так что первое из этих равновесий практически нацело сдвинуто влево. ”ксусна€ кислота электролит слабый и, следовательно, ионы —Ќ3—ќќЦ и Ќ3ќ+ образуют в основном нейтральные молекулы. ¬ результате этих двух реакций в растворе устанавливаетс€ равновесие
CH3COONa + Ќ2ќ = —Ќ3—ќќЌ + Na+ + ќЌЦ
и раствор будет иметь щелочную реакцию.
≈сли растворить в воде соль слабого основани€ и сильной кислоты, например сульфат железа (III), то можно записать следующие реакции равновеси€
Fe2(SO4)3 = 2Fe3+ + 3SO42Ц
12H2O = 6H3O+ + 6OHЦ
Fe2 (SO4)3 + 12Ќ2ќ = 2Fe (OH)3 + 6Ќ3ќ+ + 3SO42Ц
т. е. реакци€ раствора будет кисла€.
»з сказанного €сно, что в случае растворени€ в воде соли сильной кислоты и сильного основани€ нейтральна€ реакци€ среды должна сохран€тьс€. ќднако часто Ђсилыї сильного основани€ и сильной кислоты не вполне одинаковы. “огда раствор будет приобретать слабокислые или слабоосновные свойства.
–астворы, содержащие слабые кислоты и соли, образуемые этими кислотами и сильными основани€ми, или содержащие слабыеосновани€ и соли, образуемые этими основани€ми и сильными кислотами, обладают замечательным свойством противосто€ть изменению рЌ при добавлении к ним кислот или оснований. Ёто свой-ство называетс€ буферным свойством, растворов, а растворы, обладающне им, Ч буферными растворами.
¬ернемс€ к рассмотрению водного раствора, содержащего уксусную кислоту и ацетат натри€. ƒобавим к этому раствору сильное основание, например гидроксид натри€. ѕроизойдет реакци€ нейтрализации NaOH слабой кислотой
—Ќ3—ќќЌ + Na+ + ќЌЦ = —Ќ3—ќOЦ + Na+ + Ќ2O
благодар€ чему кислотность раствора практически останетс€ не-изменной или изменитс€ во много раз меньше, чем если бы мы добавили NaOH к раствору ацетата натри€.
≈сли же к буферному раствору добавить сильную кислоту, например сол€ную, то произойдет реакци€ с ацетатом натри€
—Ќз—ќOЦ + Na+ + Ќ3ќ+ + ClЦ = —Ќ3—ќќЌ + Na+ + —lЦ + Ќ2ќ
с образованием недиссоциированных молекул уксусной кислоты, т. е. с поглощением ионов гидроксони€.
»зменение концентрации ионов гидроксони€ буферного раствора в результате добавки кислого или щелочного реагента рассчитывают следующим образом. «аписывают константу равновеси€ дл€ уксусной кислоты:

¬ kвходит [Ќ2ќ]. ¬ этом уравнении концентраци€ недиссоциированных молекул очень слабо диссоциирующей уксусной кислоты практически равна аналитической концентрации кислоты (—к), а концентраци€ ацетат-ионов равна аналитической концентрации полностью диссоциирующего ацетата натри€ (—с). “огда
|
|
|
[H3O+] = KkCk/Cc
¬ буферном растворе, содержащем хлорид аммони€ и аммиак, при добавлении кислоты происходит ее нейтрализаци€ аммиаком
NH4ќЌ + Ќ3ќ+ + ClЦ= NH4+ + —lЦ + 2Ќ2ќ
а при добавлении щелочи гидроксид-ионы св€зываютс€ ионами аммони€ в недиссоциирующие молекулы аммиака:
NH4+ + —lЦ + Na+ + ќЌЦ = NH4OH + Na+ + —lЦ
онцентраци€ ионов водорода определ€етс€ из выражени€ дл€ константы равновеси€ основани€:

ѕоскольку в буферной смеси концентрацию недиссоциированных молекул аммиака можно считать равной аналитической концентрации аммиака (—0), а концентрацию NH4+ Ч аналитической концентрации хлорида аммони€ (—с), то
 и
и 
“аким образом, концентраци€ ионов водорода (и соответственно рЌ) в буферном растворе зависит от отношени€ в нем аналитических концентраций кислоты или основани€ и соли, а также от константы равновеси€. —ледовательно, при разбавлении буферного раствора его рЌ не должно измен€тьс€. Ќа самом деле небольшое изменение часто наблюдаетс€. Ёто изменение рЌ обусловлено изменением коэффициента активности соли с разведением. ѕоскольку соль в буферном растворе €вл€етс€ сильным электролитом, мы должны дл€ строгого описани€ ее поведени€ пользоватьс€ пон€тием активности, а не концентрации.
—пособность буферных растворов противосто€ть изменению рЌ количественно выражаетс€ величиной

и называетс€ буферной емкостью. Ѕуферна€ емкость Ч это количество добавл€емой кислоты или щелочи (dx), необходимое дл€ изменени€ рЌ на единицу.
ќпределение буферной емкости производитс€ следующим образом. ѕусть имеем буферный раствор, состо€щий из слабой одноосновной кислоты Ќј и ее соли с сильным основанием. ќпределим сперва концентрацию недиссоциированных молекул кислоты Ќј, ќчевидно
[HA] = Ck Ц [AЦ]
»з услови€ электронейтральности раствора имеем
[K+] +[H3O+] = [AЦ] + [OHЦ]
где [ +] Ч концентраци€ катионов в буферном растворе.
«апишем теперь выражение дл€ константы равновеси€ кислоты
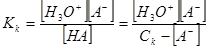
откуда
KKCK Ц KK [AЦ] = [H3O+][AЦ]
и
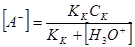
ѕодставим это выражение в уравнение электронейтральности и определим [ +]

»зменение концентрации катионов в буферном растворе может произойти только за счет концентрации добавленного основани€, т. е.
∂[K+] = ∂x
“огда

¬ скобках концентраци€ ионов гидроксони€ заменена выражением, содержащим рЌ. ѕоскольку Ц ln [Ќ3ќ+] = 2,3 рЌ, то [Ќ3ќ+] = еЦ2,3pH.
ƒифференцирование выражений в скобках по рЌ дает:

“ак как

то окончательно имеем

¬ этом выражении первый член в скобках обычно много больше второго и третьего членов. ƒействительно, например, дл€ буферного раствора —Ќ3—ќќH + CH3COONa, дл€ которого к ≈10Ц5, если примем, что —к = 1,0, то, [Ќ3ќ+] ≈10Ц4. “огда

» первый член в скобках составит примерно 10Ц1, т. е. окажетс€ на три пор€дка больше последнего члена и на дев€ть пор€дков больше второго члена. —ледовательно, с достаточной точностью можно записать:

Ѕуферна€ емкость раствора может быть различной в разных област€х кислотности. ƒл€ определени€ области кислотности максимальной буферной емкости продифференцируем последнее уравнение по рЌ:

ќбозначим
u = 2,3KKCKeЦ2,3pH; v 2 = (KK + eЦ2,3pH)2
|
|
|
“огда
du = 2,3 KKCK(Ц2,3)eЦ2,3pH
d v 2 = 2 v d v = 2 v (Ц2,3eЦ2,3pH)
—ледовательно


или

ƒл€ определени€ положени€ максимума приравн€ем правую часть уравнени€ нулю. “огда после соответствующих сокращений получим
 или KK = [H3O+]; pKK = pH
или KK = [H3O+]; pKK = pH
—ледовательно, максимум буферной емкости раствора будет при том значении рЌ, которое численно равно показателю константы равновеси€ кислоты. Ќа основе этого вывода подбирают буферные растворы, обладающие наибольшим буферным действием в заданном интервале рЌ.






