Ёлектролитами называют вещества, растворы и расплавы которых про-
вод€т электрический ток.
электролитам относ€тс€ неорганические кислоты, а также основани€, амфотерные гидроксиды и соли. ќни распадаютс€ в водных растворах и расплавах на катионы ( n+) и анионы (јm-).
ѕроцесс распада молекул электролитов на ионы в среде раство≠рител€ получил название электролитической диссоциации (или ионизации).
ƒл€ количественной характеристики силы электролита используют пон€тие степени электролитической диссоциации (ионизации) - α, котора€ равна отношению числа молекул, распавшихс€ на ионы (n), к общему числу молекул электролита, введенных в раствор (N):
α = n / N.
“акимобразом, α выражаютв дол€х единицы.
ѕо степени диссоциации электролиты условно подраздел€ют на сильные (αї 1) и слабые (α <0,3).
—ильные электролиты
Ј —оли (средние, кислые, основные): ј12(SO4)3, NаHCO3, —uќЌ—l.
Ј Ќеорганические кислоты: ЌNO3, H2SO4, Ќ—1, Ќ¬г, ЌI, Ќ—lќ4. и др.
Ј √идроксиды щелочных и щелочноземельных металлов: ќЌ, NаќЌ, —а(ќЌ)2, ¬а(ќЌ)2 и др.
—ильные электролиты диссоциируют в водном растворе практически нацело: ј12(SO4)3 = 2ј13++3 SO42Ц NаHCO3 = Nа+ +Ќ—ќ3Ц
ЌNќ3 = H++Nќ3Ц Ќ2SO4 = 2Ќ++Sќ42Ц
—uќЌ—l = CuOH++ClЦ ¬а(ќЌ)2 = ¬а2++2ќЌЦ
—лабые электролиты
Ј ѕочти все органические кислоты: CH3COOH, H2C2O4 и др..
Ј Ќекоторые неорганические кислоты: H2CO3, H2S, HCN, H2SiO3, HNO2,
H2SO3, H3PO4, HClO и др.
Ј √идроксиды металлов основного характера (кроме щелочных и щелочноземельных) и гидроксид аммони€ NH4OH.
Ј јмфотерные гидроксиды: Al(OH)3, Zn(OH)2, Cr(OH)3, Sn(OH)2, Pb(OH)2 и др.
ƒл€ слабых электролитов диссоциаци€ Ц обратимый процесс, дл€ которого справедливы общие законы равновеси€.
ƒиссоциацию слабых электролитов характеризует константа равновеси€, называема€ константой диссоциации (ионизации) ƒ (табл.ѕ.3):
CH3COOH 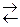 CH3COOЦ + H+
CH3COOЦ + H+


ћногоосновные кислоты и многокислотные основани€ диссоциируют ступенчато, и каждую ступень равновесного состо€ни€ характеризует сво€ константа диссоциации (причем д1 всегда больше д2 и т.д.), например при диссоциации H2S:1-€ ступень H2S 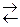 H+ + HSЦ
H+ + HSЦ  6ּ10-8;
6ּ10-8;
2-€ ступень HSЦ 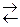 H+ + S2-
H+ + S2-  1Ј10-14,
1Ј10-14,
где [ ] ─ равновесные концентрации ионов и молекул.
ƒиссоциаци€ —u(OH)2:
1-€ ступень —u(OH)2 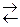 Cu(OH)+ + OH Ц
Cu(OH)+ + OH Ц
2-€ ступень Cu(OH)+ 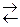 Cu2+ + OH Ц
Cu2+ + OH Ц
јмфотерные гидроксиды, напримерPb(OH)2,диссоциируют по основному типу: Pb(OH)2 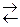 PbOH+ + OH Ц
PbOH+ + OH Ц
PbOH+ 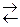 Pb2+ + OHЦ
Pb2+ + OHЦ
и кислотному: H2PbO2 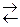 H+ + HPbO2Ц
H+ + HPbO2Ц
HPbO2Ц 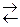 H+ + PbO22 Ц
H+ + PbO22 Ц
¬ растворах электролитов реакции протекают между ионами. ƒл€ записи ионных реакций примен€ют ионные уравнени€. ѕри составлении ионных уравнений реакций все слабые электролиты, газы и труднорастворимые электролиты записывают в молекул€рной форме, все сильные электролиты (кроме труднорастворимых солей) в ион≠ной форме. ѕримеры составлени€ ионных уравнений реакций:
Ј образование труднорастворимых соединений:
–b(Nќ3)2 + 2 I = ¯–bI2 + 2 Nќ3 –b2+ +2I Ц = ¯–bI2
|
|
|
Ј реакции с участием слабодиссоциирующих соединений:
—Ќ3—ќќNa + Ќ—1 = —Ќ3COOH + Nа—1
—Ќ3COO Ц + Ќ+ = —Ќ3COOH
Ќ—1 + NаќЌ = Nа—1 + Ќ2O Ќ+ + ќЌ Ц = Ќ2O
Ќ—1 + NЌ4OЌ = NЌ4—1+ H2O Ќ+ + NH4OH =NH4+ + Ќ2O
—Ќ3COOH +NЌ4OЌ = —Ќ3COONH4 + Ќ2ќ
—Ќ3COOH + NЌ4OЌ = CЌ3COO Ц + NH4+ + Ќ2O
Ј образование газообразных веществ:
Nа2—ќ3 + 2Ќ—1 = 2Nа—1 + —ќ2≠ + Ќ2ќ —ќ32Ц+ 2Ќ+ = —O2≠+ Ќ2O
ѕример 1. ќсуществить превращени€ NаќЌ Ѓ NаЌSќ3 Ѓ Nа2SO3.
–ешение. NаќЌ + Ќ2SO3 = NаЌSO3 + Ќ2O
ќЌЦ + Ќ2SO3 = ЌSќ3Ц +Ќ2ќ
NаHSO3 +NаќЌ = Nа2SO3 + Ќ2O
ЌSќ3Ц + ќЌ Ц = SO32 Ц + Ќ2ќ
ѕример 2. ќсуществить превращени€ Ni(ќЌ)2 Ѓ (NiOH)2SO4 Ѓ NiSO4.
–ешение. 2Ni(ќH)2 + Ќ2SO4 = (NiOЌ)2SO4 + Ќ2O
¯2Ni(ќЌ)2 + 2Ќ+ + SO42 Ц = ¯(NiќЌ)2SO4 + Ќ2O
¯(NiќЌ)2SO4 + Ќ2SO4 = 2NiSO4 + 2Ќ2ќ
¯(NiOЌ)2SO4 + 2Ќ+ = 2Ni 2+ + 2SO42Ц + 2Ќ2ќ
¬нимание! ќсновные соли, как правило, нерастворимы в воде, поэтому при написании ионных уравнений их не расписывают на ионы.
«адани€ к подразделу 3.2
«адани€ 121-140. Ќапишите в молекул€рной и ионной формах уравнени€ возможных реакций предложенных оксидов с H2O, Na2O, KOH, HNO3.
| 121.N2O3; Na2O | 126.SO2; CuO | 131.MnO; P2O5 | 136.N2O5; CuO |
| 122.SnO; P2O5 | 127.Cr2O3; Cl2O7 | 132.BaO; Mn2O7 | 137.P2O5; CoO |
| 123.SO3; CaO | 128.CoO; ZnO | 133.CdO; SnO | 138.PbO; MgO |
| 124.SiO2; NiO | 129.P2O3; FeO | 134.As2O5; CuO | 139.Cl2O7; MnO |
| 125.PbO; N2O5 | 130.Fe2O3; K2O | 135.Al2O3; SiO2 | 140.SO3; TiO |
«адани€ 141-160. Ќапишите дл€ предложенных соединений уравнени€ диссоциации, а также в молекул€рной и ионной формах уравнени€ возможных реакций взаимодействи€ их с H2SO4 и NaOH.
| 141.HCl; Cr(OH)3 | 151.Ca(OH)2; H3PO4 |
| 142.Cd(OH)2; H2S | 152.HNO3; Be(OH)2 |
| 143.Cu(OH)2; HBr | 153.H2—r2O7; KOH |
| 144.H2SO3; Sn(OH)2 | 154.HCN; Ga(OH)3 |
| 145.H2SiO3; Pb(OH)2 | 155.KOH; H2CO3 |
| 146.CH3COOH; Fe(OH)3 | 156.HF; Be(OH)2 |
| 147.H2Se; Zn(OH)2 | 157.NH4OH; HClO4 |
| 148.Fe(OH)2; H3AsO3 | 158.Pb(OH)2; HNO2 |
| 149.RbOH; HI | 159.Mg(OH)2; HClO |
| 150.H2Te; Al(OH)3 | 160.Ga(OH)3; HMnO4 |
«адани€ 161-180. Ќапишите уравнени€ диссоциации солей и назовите их.
| 161.ZnCl2, MnOHCl, Ba(HSO3)2 | 171.Pb(HSO4)2, NH4NO3, CoOHCl |
| 162.K2HAsO3, AlOHCl2, Na2SO3 | 172.Al(OH)2NO3, Fe2(SO4)3, KHSe |
| 163.KHSO3, (PbOH)2SO4, CrBr3 | 173.CsHTe, Ca3(PO4)2, MnOHBr |
| 164.Fe(NO3)3, SnOHCl, NaHTe | 174.Mn(NO3)2, Bi(OH)2Cl, KHS |
| 165.NaHSe, CoOHNO3, MgCl2 | 175.Al2(SO4)3, CrOHCl2, KHSO3 |
| 166.CdOHBr, NiCl2, KH2PO4 | 176.NaHSe, NiOHNO3, ZnSO4 |
| 167.CaBr2, (SnOH)2SO4, K2HPO4. | 177.CrOHSO4, BaBr2, CsHSO3 |
| 168.BaCl2, Ca(HCO3)2, AlOHCl2 | 178.Cu(NO3 )2, CoOHCl, NaHS |
| 169.NiBr2, (CoOH)2SO4, KHCO3. | 179.FeCl2, NaH2AsO4, KCrO2 |
| 170.NiOHCl, NiBr2, NaH2PO4 | 180.AlOHBr2, Sr(HS)2, K2SO3 |
«адани€ 181-200. Ќапишите в молекул€рной и ионной формах уравнени€ реакций дл€ следующих превращений.
181. Ni(OH)2 (NiOH)2SO4 NiSO4 Ni(OH)2; H3PO4 KH2PO4
182. CuSO4 (CuOH)2SO4 Cu(OH)2 CuOHNO3; NaHSO3 Na2SO3
183. Bi(NO3 )3 BiOH(NO3)2 Bi(OH)3 Bi2O3; Ca3(PO4)2 Ca3(H2PO4)2
184. Co(OH)2 CoOHCl CoCl2 Co(NO3)2; NaOH NaHSO3
185. Pb(NO3 )2 PbOHNO3 Pb(OH)2 K2PbO2; Na2Te NaHTe
|
|
|
186. NiCl2 Ni(OH)2 NiOHCl NiCl2; Ba(HS)2 BaS
187. CrOHCl2 CrCl3 Cr(OH)3 CrOHSO4; H2SiO3 NaHSiO3
188. (SnOH)2SO4 SnSO4 Sn(OH)2 Na2SnO2; K2SO3 KHSO3
189. NiBr2 NiOHBr Ni(OH)2 NiSO4; NaHSiO3 Na2SiO3
190. CoSO4 Co(OH)2 (CoOH)2SO4 Co(NO3)2; H2S Ca(HS)2
191. Cr2(SO4)3 CrOHSO4 Cr2(SO4)3 CrCl3; Mg3(PO4)2 MgHPO4
192. NiSO4 (NiOH)2SO4 Ni(OH)2 NiBr2; NaHCO3 Na2CO3
193. FeOHSO4 Fe2(SO4)3 Fe(OH)3 FeCl3; MgCO3 Mg(HCO3)2
194. Sn(OH)2 SnOH—l K2SnO2 Sn(OH)2; H3AsO4 KH2AsO4
195. NiBr2 Ni(OH)2 NiOHCl NiCl2; BaSO3 Ba(HSO3)2
196. Al(OH)3 Al(OH)2Cl AlCl3 Al(NO3)3; NaH2AsO3 Na3AsO3
197. CoCl2 Co(OH)2 (CoOH)2SO4 CoSO4; H2CO3 NaHCO3
198. Bi(OH)3 Bi(OH)2NO3 Bi(OH)3 Bi2O3; K2HPO4 H3PO4
199. Cu(OH)2 CuOHCl CuCl2 Cu(NO3)2; H2Se KHSe
200. CoSO4 (CoOH)2SO4 Co(OH)2 Co(NO3)2; K2SO3 KHSO3
√идролиз солей
√идролиз солей Ц это процесс взаимодействи€ ионов соли с молекулами воды, привод€щий к смещению ионного равновеси€ воды и изменению рЌ среды.
√идролиз €вл€етс€ обратимым процессом. ¬ реакци€х гидролиза участву-
ют ионы слабых электролитов: катионы слабых оснований и анионы слабых кислот. ѕричина гидролиза Ц образование слабодиссоциированных или труднорастворимых продуктов. —ледствием гидролиза €вл€етс€ нарушение равновеси€ в системе H2O 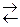 H+ + OHЧ ; в результате среда становитс€ либо кислой (рЌ < 7), либо щелочной (pH > 7).
H+ + OHЧ ; в результате среда становитс€ либо кислой (рЌ < 7), либо щелочной (pH > 7).
Ј —оль, образованна€ сильным основанием и слабой кислотой, подвергаетс€ гидролизу по аниону. –еакци€ среды щелочна€ (pH > 7). ѕерва€ ступень гидролиза: Na2CO3 + HOH 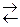 NaHCO3 + NaOH; CO32Ч + HOH
NaHCO3 + NaOH; CO32Ч + HOH 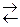 HCO3Ц + OHЧ
HCO3Ц + OHЧ
Ј —оль, образованна€ слабым основанием и сильной кислотой, подвергаетс€ гидролизу по катиону. –еакци€ среды кисла€ (pH < 7).
ѕерва€ ступень гидролиза:
Cu(NO3)2 + HOH 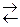 CuOHNO3 + HNO3 Cu2+ + HOH
CuOHNO3 + HNO3 Cu2+ + HOH 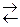 CuOH+ + H+
CuOH+ + H+
Ј —оль, образованна€ слабым основанием и слабой кислотой, подвергаетс€ гидролизу по катиону и аниону. ’арактер среды определ€етс€ константами диссоциации образовавшихс€ слабых электролитов.
CH3COONH4 + HOH 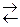 CH3COOH + NH4OH
CH3COOH + NH4OH
CH3COOЧ + NH4+ + HOH 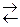 CH3COOH + NH4OH
CH3COOH + NH4OH
Ј ѕри совместном гидролизе двух солей образуютс€ слабое основание и слаба€ кислота: 2FeCl3 + 3Na2S +6H2O = 2Fe(OH)3 ¯ + 3H2S≠ + 6NaCl
2Fe3+ + 2S2Ч + 6H2O = 2Fe(OH)3 ¯ + 3H2S≠
Ј —оль, образованна€ сильной кислотой и сильным основанием, гидролизу
не подвергаетс€, реакци€ среды нейтральна€: KNO3 + HOH ¹
»оны K+ и NO3Ч не образуют с водой слабодиссоциирующих продуктов (KOH и HNO3 Ц сильные электролиты).
«адани€ к подразделу 3.3
«адани€ 201-220. Ќапишите в молекул€рной и ионной формах уравнени€ реакций гидролиза солей, укажите значени€ рЌ растворов этих солей (больше или меньше семи).
| 201.NaNO2, Cu(NO3)2 | 211.Na2HPO4, Mg(NO3)2 |
| 202.AlCl3, NaHCO3 | 212.Al2 (SO4)3, Na2SeO3 |
| 203.Na3PO4, ZnCl2 | 213.CuSO4, K3PO4 |
| 204.FeCl2, K2S | 214.Na2SO3, Fe2 (SO4)3 |
| 205.K2SO3, ZnSO4 | 215.NaCN, FeSO4 |
| 206.NH4Cl, KClO | 216.Ba(CH3COO)2, CoSO4 |
| 207.Na2Se, MnCl2 | 217.NiSO4, NaF |
| 208.ZnSO4, BaS | 218.Pb(NO3)2, Ba(NO2)2 |
| 209.Ni (NO3)2, KNO2 | 219.Cr2(SO4)3, Na CH3COO |
| 210.NH4Br, Na2S | 220.KHS, MgSO4 |
«адани€ 221-240. Ќапишите в молекул€рной и ионной формах уравнени€ реакций совместного гидролиза предложенных солей.
| 221.Fe2(SO4)3 + Na2CO3 | 231.CrCl3 + K2S |
| 222.Na2S + Al2 (SO4)3 | 232.Na2CO3 + Cr (NO3)3 |
| 223.NH4Cl + Na2SiO3 | 233.K2SiO3 + Bi (NO3)3 |
| 224.Cr2 (SO4)3 + K2S. | 234.Na2SO3 + CrCl3 |
| 225.K2CO3 + Bi (NO3)3 | 235.NH4NO3 + Na2SiO3 |
| 226.Na2S + AlCl3 | 236.AlCl3 + Na2SO3 |
| 227.BeSO4 + K2S | 237.K2SO3 + CrCl3 |
| 228.Cr2 (SO4)3 + Na2SO3 | 238.Na2S + Al2 (SO4)3 |
| 229.K2SO3 + AlBr3 | 239.Fe (NO3)3 + K2CO3 |
| 230.Bi (NO3)3 + Na2CO3 | 240.Al2 (SO4)3 + Na2CO3 |
|
|
|






