¬ведение
’имические реакции и закон действующих масс. –авновесный состав Ц совокупность равновесных концентраций. »х обозначают формулами реагентов в квадратных скобках, [A j ], например: [H+], [H3PO4], [PO43‑]. –ассчитыва€ его, в соотношени€х удобно приписывать стехиометрическим коэффициентам в реакци€х знаки, которые получаем, если реакции переделать на алгебраические уравнени€, перенос€ все реагенты вправо и оставл€€ влевой части 0. Ќапример, реакции
H2O Û H+ + OH‑, H3PO4 Û 3 H+ + PO43‑
переход€т в
0 Û ‑ H2O + H+ + OH‑, 0 Û ‑ H3PO4 + 3 H+ + PO43‑.
ќбщий вид реакции между A j - молекулами, ионами, структурными фрагментами (такими, как активные центры макромолекул) -
0 Û 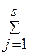 a j A j,
a j A j,
где a j - стехиометрический коэффициент, положительный дл€ продуктов реакции, отрицательный дл€ исходных веществ и равный 0 дл€ не реагентов, которые не принимают участие в реакции. »ндекс j, номер реагента, не имеет отношени€ к стехиометрическому составу, в отличие от стехиометрических индексов в химических формулах, таких как Br2 или I3‑.
”слови€ равновеси€ реакции задает закон действи€ масс («ƒћ),
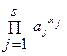 = K,
= K,
где aj = a (A j) - активность реагента A j, K - термодинамическа€ константа равновеси€. јктивности св€заны с равновесными концентраци€ми,
aj = g j [A j ],
где коэффициент активности g j учитывает вли€ние на энергетическое состо€ние реагента тех физических и химических взаимодействий, которые не отображены в реакци€х. оэффициент g j медленно мен€етс€ с составом системы. ѕодставл€€ в «ƒћ выражение aj через [A j ], приходим к соотношению, аналогичное «ƒћ, но с равновесными концентраци€ми вместо активностей.
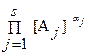 = K c = K /
= K c = K / 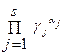 , lg K c = lg K ‑
, lg K c = lg K ‑ 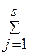 a j lg g j.
a j lg g j.
“ак называема€ концентрационна€ константа равновеси€, K c, зависит от состава раствора, поскольку от этого завис€т g j. „асто использу€ K c, индекс "c" можно и не указывать. ¬ справочниках привод€т как термодинамические, так и концентрационные константы.
≈стественно, что aj = 0 при [A j ] = 0. роме того, можно измен€ть масштаб активности, не измен€€ форму уравнений «ƒћ и других соотношений термодинамики. ¬ыбор масштаба может облегчить работу. ќдин из способов выбора - задать величину активности дл€ определенного состава системы (точку на шкале активностей). “ак, выбор aj = 1 дл€ конденсированных фаз - химических индивидов ≥ чистого растворител€ - упрощает уравнение «ƒћ, так как эту активность в системах с такими фазами в выражении «ƒћ вообще не записывают. ƒл€ исключени€ ошибок в записи «ƒћ, необходимо в реакци€х указывать фазовую принадлежность, например, BaSO4(s), где s - сокращенно "solid" - твердый, или Hg(l), где l - сокращенно "liquid" - жидкий. ƒл€ смесей газов активности приравнивают к парциальному давлению в атмосферах (атм). Ётот выбор единиц сохранили, чтобы не мен€ть фонды справочных данных.
ƒругой выбор активностей - приблизить их значени€ к равновесным концентраци€м дл€ бесконечно разбавленного раствора, где [A j ] растворенных веществ стремитс€ к 0. —читают, что тогда коэффициенты активности стрем€тс€ к 1,
|
|
|
g j Ѓ 1, aj Ѓ [A j ], если все [A j ] Ѓ 0.
«ависимость коэффициентов активности от состава раствора описывают полуэмпирическими формулами. Ќа g j ионов наиболее вли€ют ион - ионные взаимодействи€. »х обычно учитывают через ионную силу раствора,
I = {S zj 2 [A j ]} / 2,
где zj - зар€д частицы A j в атомных единицах. ѕриблизительно оценива€ ионную силу, ограничиваютс€ вкладом от ионов сильных электролитов (тех, которые полностью продиссоциировали), если они преобладают в растворе. »сследу€ равновеси€, из сильных электролитов образуют так называемый "солевой фон", что стабилизирует коэффициенты активности. ¬ водных растворах сильными €вл€ютс€ кислоты HCl, HClO4, HBr, HI, H2SO4 (по первой ступени диссоциации), HNO3; основани€ и соли с катионами Na+, K+, Rb+, Cs+, не склонными к комплексообразованию, и соли с анионом ClO4‑.
ƒл€ водных растворов примен€ем формулу ƒэвиса,
lg g j = zj 2 lg g, lg g = ‑ 0,5 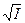 / (1 +
/ (1 + 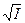 ) + 0,15 I,
) + 0,15 I,
модификацию формулы ƒеба€-√юкел€. ќна тем точнее, чем меньшей €вл€етс€ ионна€ сила. ≈сли I > 0,5, то применимость формулы дл€ точных расчетов довольно сомнительна. ѕодставл€€ эту формулу в выражение логарифма концентрационной константы при заданной ионной силе, получаем
lg K c = lg K ‑ { 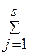 a j zj 2} lg g.
a j zj 2} lg g.
¬ справочниках обычно указывают ионную силу раствора или даже состав ионной среды, к которым относ€тс€ значени€ константы. ¬этих учебных таблицах обычно, если не указаны другие услови€, приведены по два значени€ в отдельных р€дах: верхнем - дл€ "термодинамической константы" (при I = 0), нижнем - при I = 1, где формулой ƒэвиса пользоватьс€ нежелательно. —тандартной температурой €вл€етс€ 25о—. ƒанные на другие температуры пересчитываем по формуле
lg K (t, oC) = lg K (25 oC) + 5,88×10‑4×(t Ц 25)× D H,
где D H - энтальпи€ реакции, котора€ подаетс€ в таблицах в ƒж/моль.
“ипы реакций. „тобы различать константы, ћеждународным союзом по чистой и прикладной химии (International Union of Pure and Applied Chemistry, IUPAC) рекомендовано после символа в круглых скобках записывать реакции, например,
K (H3PO4 Û 3 H+ + PO43‑), или K (0 Û ‑ H3PO4 + 3 H+ + PO43‑).
„тобы сократить обозначени€, дл€ самых распространенных типов реакций рекомендованы символы констант «ƒћ, которые основаны на практике авторитетных справочников. »спользуют две буквы, K и , с различными индексами. ѕервый из индексов указывает тип реакции: дл€ комплексообразовани€ он отсутствует, дл€ присоединени€ протона H+ к основани€м - буква H, дл€ его отщеплени€ от кислот - буква a (от "acidity" - кислотность), дл€ растворимости - буква s (от "solubility" - растворимость), дл€ распределени€ между двум€ растворител€ми - буква D (от "distribution" - распределение). ќстальные индексы в константах указывают стехиометрический состав продукта. ѕодробности - в таблице, где M - комплексообразователь (сокращение от "металл", хот€ он может и не быть ионом металла, L - лиганд. ќбозначение L применено и в общей записи кислотно-основных превращений, т.к. типичные лиганды €вл€ютс€ основани€ми Ѕренстеда.
ќкислительно - восстановительные полуреакции - это передача электронов между окисленной и восстановительной формами. ¬ стандартной записи окисленна€ форма (окислитель) и электроны €вл€ютс€ исходными веществами, а восстановленна€ форма (восстановитель) €вл€ютс€ продуктами, например,
|
|
|
Cr3+ + e‑ Û Cr2+, S4O62‑ + 2 e‑ Û 2 S2O32‑, 2 Hg2+ + 2 e‑ Û Hg22+, HCrO4‑ + 7 H+ + 3 e‑ Û Cr3+ + 4 H2O, гZn2+ + 2 e‑ Û Zn(s).
–оль электронов в полуреакции аналогична€ рол€м лигандов в комплексообразовании и ионов H+ в кислотно-основных реакци€х. ≈сли мерой основных свойств раствора €вл€етс€ pH, то мерой окислительной способности - окислительный потенциал E. ќн €вл€етс€ пропорциональным показателем активности электронов pe = ‑ lg a (e),
E = q pe = ‑ q lg a (e).
оэффициентом пропорциональности €вл€етс€ множитель Ќернста
q = ln (10) R T / F, (q = 0,05916 ¬ при 25 o—),
где ln (10) = 2,3026, R - газова€ посто€нна€, T - абсолютна€ температура, F - посто€нна€ ‘араде€. Ўкалу активностей электрона выбирают таким образом, чтобы 1 константа «ƒћ была равна полуреакции
2 H+ + 2 e‑ Û H2(g), lg K = 0.
”словие равновеси€ полуреакции
0 Û ae e- + 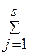 a j A j, lg K,
a j A j, lg K,
задают уравнением Ќернста
E = E 0 ‑ (q / |ae|) 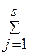 a j lg aj = E 0 + (q / ae)
a j lg aj = E 0 + (q / ae) 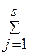 a j lg aj.
a j lg aj.
¬ соответствии с правилом о знаках, стехиометрический коэффициент при е‑ €вл€етс€ отрицательным.
ќсновные типы реакций и обозначени€ их констант
–авновесий
| –еакции (общий вид и примеры) | ќбозначени€ | ѕримечани€ |
| 1. онстанты устойчивости комплексов | ||
| m M + n L Û M m L n | b nm | ќбщие константы устойчивости |
| 2 Ag+ + 6 I‑ Û Ag2I64‑ | b62 | |
| M + n L Û ML n | b n | ƒл€ одно€дерных комплексов m = 1 опускают |
| Ag+ + NH3 Û AgNH3+ | b1 | |
| Ag+ + 2 NH3 Û Ag(NH3)2+ | b2 | |
| ML n ‑1 + L Û ML n | Kn | K Ц ступенчатые константы стойкости, Kn = b n / b n ‑1 |
| Ag+ + NH3 Û AgNH3+ | K 1 | |
| AgNH3+ + NH3 Û Ag(NH3)2+ | K 2 | |
| 2. онстанты кислотно-основных равновесий | ||
H2O Û H+ + OH‑ 
| Kw | »онное произведение воды |
| m H+ + L Û H m L | bH m | ≈сли L Ц лиганд, H+ комплексообразователь, то bH m Ц це b1 m |
| H+ + PO43‑ Û HPO42‑ | bH1 | |
| 2 H+ + PO43‑ Û H2PO4‑ | bH2 | |
| 3 H+ + PO43‑ Û H3PO4 | bH3 | |
| H+ + H m ‑1L Û H m L | K H m | K H m Ц ступенчатые константы стойкости K H m = bH m / bH(m ‑1) |
| H+ + PO43‑ Û HPO42‑ | K H1 | |
| H+ + HPO42‑ Û H2PO4‑ | K H2 | |
| H+ + H2PO4‑ Û H3PO4 | K H3 | |
| H m +1L Û H m L + H+ | Ka (N ‑ m) | —тупенчата€ константа ионизации кислоты H N L, Ka (N‑m) = 1 / K H(m +1) |
| H3PO4 Û H+ + H2PO4‑ | Ka 1 | |
| H2PO4‑ Û H+ + HPO42‑ | Ka 2 | |
| HPO42‑ Û H+ + PO43‑ | Ka 3 | |
| m M + n OH‑ Û M m (OH) n | b nm | онстанты стойкости гидроксокомплексов (отдельного случа€ комплексов) |
| 2 Fe3+ + 2 OH‑ Û Fe2(OH)24+ | b22 | |
| Hg2+ + OH‑ Û HgOH+ | b1 | |
| Hg2+ + 2 OH‑ Û Hg(OH)2 | b2 | |
| Hg2+ + 3 OH‑ Û Hg(OH)3‑ | b3 | |
| m M + n H2O Û M m (OH) n + n H+ | *b nm | онстанта взаимодействи€ иона металла с H2O *b nm = b nm K wn, m = 1 опускают |
| 2 Fe3+ + 2 H2O Û Fe2(OH)24+ + 2 H+ | *b22 | |
| M + n H2O Û M(OH) n + n H+ | *b n | |
| Hg2+ + H2O Û HgOH+ + H+ | *b1 | |
| Hg2+ + 2 H2O Û Hg(OH)2 + 2 H+ | *b2 | |
| Hg2+ + 3 H2O Û Hg(OH)3‑ + 3 H+ | *b3 |
| 3. онстанты гетерогенных реакций | ||
L Û L(орг), или L Û 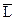 , ,
| K D | онстанта распределени€ (в скобках - растворитель) |
Br2 Û Br2(CCl4), или Br2 Û 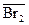
| ||
| Al3+ + 3 HOxin (орг) Û Û AlOxin3 (орг)+ 3 H+ | K ex | ќбща€ константа экстракции |
| L(g) Û L | Kp | «акон √енри, p в атмосферах |
| CO2(g) Û CO2 | ||
| L(s) Û L, L(l) Û L, | Ks | –астворимость (s Ц твердое, l Ц жидкое вещество) |
| I2(s) Û I2, Br2(l) Û Br2, | ||
| M m L n (s) Û m ML q + (n Ц m q) L | Ksq | ѕроизведение растворимости (q = 0 можно опускать), Ksq = Ks 0 b q. Ќиже - сложный случай, где вслед за Ks в скобках приведены продукты в растворе. |
| HgI2(s) Û Hg2+ + 2 I‑ | Ks 0 | |
| HgI2(s) Û HgI+ + I‑ | Ks 1 | |
| HgI2(s) Û HgI2 | Ks 2 | |
| HgI2(s) + I‑ Û HgI3‑ | Ks 3 | |
| HgI2(s) + 2 I‑ Û HgI42‑ | Ks 4 | |
| Cu3(OH)2(CO3)2(s) Û 3 Cu2+ + 2 OH‑ + 2 CO32‑, Ks (3 Cu2+, 2 OH‑, 2 CO32‑) |
”равнением «ƒћ, прологарифмированным и умноженным на |ae|, €вл€етс€
|
|
|
‑ ae pe + 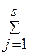 a j lg aj = lg K = E 0 |ae| / q = ‑ E 0 ae / q.
a j lg aj = lg K = E 0 |ae| / q = ‑ E 0 ae / q.
Ћинейные комбинации реакций. –ассчитыва€ равновесный состав, мы как правило вынуждены учитывать много различных реакций. ¬место того, чтобы преобразовывать алгебраические уравнени€ «ƒћ, часто нагл€днее и удобнее превращать реакции, дл€ которых существуют справочные данные, переход€ к линейным комбинаци€м. »х получаем, и как линейную комбинацию векторов в математике, умножа€ исходные реакции на числа, а затем прибавл€€ их. Ќекоторые реагенты УсокращаемФ - превращаем в 0 коэффициенты при них. ѕри комбинации r реакций, сокращаем по крайней мере (r ‑ 1) реагентов.
ѕример. —оставл€ем уравнение реакции
Fe3+ + 2 F‑ Û FeF2+, lg b2 = 9,30; H+ + F‑ Û HF, lg K H = 3,17,
линейную комбинацию, в которую не входит F‑. Ёто оформим таким образом,
| Fe3+ + 2 F‑ | Û | FeF2+, | lg b2 = 9,30, | |
| ‑ 2 | H+ + F‑ | Û | HF, | lg K H = 3,17 |
| Fe3+ + 2 HF | Û | FeF2+, + 2 H+, | lg b2 Ц 2 lg K H = 2,96, |
где слева - множители при линейной комбинации. —оответствующа€ линейна€ комбинаци€ логарифмов констант «ƒћ - это
| ‑ lg a (Fe3+) ‑ 2 lg a (F‑) + lg a (FeF2+) = lg b2 = 9,30, | |
| ‑2 | ‑ lg a (H+) ‑ lg a (F‑) + lg a (HF) = lg K H = 3,17, |
‑ lg a (F‑) ‑ 2 lg a (HF) + lg a (FeF2+) + 2 lg a (H+) =
= lg b2 Ц 2 lg K H = 2,96.
ќбщее правило: логарифм константы линейной комбинации реакций равн€етс€ аналогичной (то есть с этими же коэффициентами) линейной комбинации логарифмов констант исходных реакций
ѕор€док подачи материала в таблицах. ћатериал сгруппирован по лигандам в таком пор€дке: неорганические лиганды (ќЌ‑, далее - по символам элементов, которые образуют лиганд, по алфавиту, а в пределах одного элемента Ц в соответствии с увеличением его окислительного числа); органические лиганды - амины, карбоновые кислоты, аминокислоты, другие (в пор€дке возрастани€ числа атомов элементов по номенклатуре Ѕельштейна); окислительно-восстановительные полуреакции, индикаторы. ѕор€док комплексообразователей: сначала Ќ+, остальные Ц по алфавиту символов элементов.
Ћитература
| 1. | Marcus Y. Recommended symbols for solution equilibria. Analytical chemistry division. Commission of equilibrium data. // Pure Appl. Chem., 1959, v. 18, No. 3, р. 459-464. |
| 2. | Martell A. E., Smith R. M. Critical stability constants. New York: Plenum. V. 1 Amino acids. 1974. 496 р.; V. 2. Amines. 1975. 415 р.; V. 3. Other organic ligands. 1977. 495 р.; V. 4. Inorganic complexes. 1976. 257 р.; V. 5. First supplement. 1982. 604 р. |
| 3. | Kotrlý S., Šůcha L. Handbook of chemical equilibria in analytical chemistry. New York: Ellis, 1985. 414 р. |
| 4. | Stary J., Freiser H. Equilibrium constants of liquid-liquid distribution reactions. Part IV. Chelating extractants. Oxford et al: Pergamon, 1978. 228 р. |
| 5. | умок ¬.Ќ., улешова ќ.ћ., арабин Ћ.ј. ѕроизведени€ растворимости. Ќовосибирск: Ќаука, 1983. - 257 —. |
|
|
|






