»ќЌЌќ-ћќЋ≈ ”Ћя–Ќџ≈ ”–ј¬Ќ≈Ќ»я
”–ќ¬≈Ќ№ ¬
1. ¬ычислить рЌ следующих водных растворов:
ј) 0,02 ћ HCl,
Ѕ) 0,2 ћ KOH.
| ƒано: c HCl = 0,02 ћ c KOH = 0,2 ћ | –ешение
а) HCl = H+ + Cl-
рЌ = -lg c 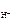 ; ;
|
| pH Ц? |
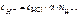
HCl Ц сильный электролит, α = 1,
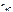 Ц число Ќ+, образовавшихс€ при диссоциации одной молекулы Ќ—l,
Ц число Ќ+, образовавшихс€ при диссоциации одной молекулы Ќ—l,
n 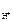 = 1, тогда
= 1, тогда 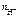 = c
= c 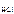 = 0,02 моль/л = 2 Ј 10-2 моль/л.
= 0,02 моль/л = 2 Ј 10-2 моль/л.
рЌ = -lg2Ј10-2 = -lg2 + (-lg10-2)= Ц 0,3 + 2 = 1,7.
б) KOH = + + ќЌ-;
рЌ = 14 Ц рќЌ; рќЌ = -lg c 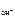 ,
,
c 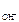 = c
= c 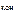 Ј α Ј n
Ј α Ј n 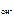 ,
,
ќЌ Ц сильный электролит, α = 1,
n 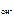 Ц число ќЌ-, образовавшихс€ при диссоциации одной молекулы ќЌ, n
Ц число ќЌ-, образовавшихс€ при диссоциации одной молекулы ќЌ, n 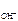 = 1, тогда c
= 1, тогда c 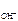 = c KOH = 2Ј10-1 моль/л,
= c KOH = 2Ј10-1 моль/л,
рќЌ = Цlg(2Ј10-1) = Цlg2 + (Цlg10-1) = Ц 0,3 + 1 = 0,7.
рЌ = 14 Ц 0,7 = 13,3.
ќтвет: рЌ 0,02 ћ HCl равно 1,7;
–Ќ 0,2 ћ ќЌ равно 13,3.
¬ычислить рЌ 0,05 ћ водного раствора хлорноватистой кислоты (HOCl).
ƒано:
c 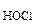 = 0,05 ћ = 0,05 ћ
| –ешение
HOCl <=> H+ + OCl-
HOCl Ц слабый электролит.
рЌ = Цlg[H+]; [H+] = c ЌO—lЈαЈ n 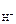 , ,
|
| pH Ц? |
n 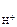 Ц число Ќ+ образовавшихс€ при диссоциации одной молекулы HOCl, n
Ц число Ќ+ образовавшихс€ при диссоциации одной молекулы HOCl, n 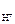 = 1.
= 1.
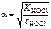 ;
;
HOCl Ц константа диссоциации HOCl:
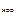 = 5,0Ј10-8 (таблица),
= 5,0Ј10-8 (таблица),
“огда
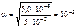
c 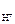 = 5Ј10-2 Ј 10-3 Ј 1 = 5,0 Ј 10-5 моль/л,
= 5Ј10-2 Ј 10-3 Ј 1 = 5,0 Ј 10-5 моль/л,
тогда рЌ = Ц lg5,0Ј10-5 = (-lg 5) + (-lg10-5) = Ц 0,7 + 5 = 4,3.
ќтвет: рЌ 0,05 ћ HOCl равно 4,3.
3. ќпределить произведение растворимости MgF2, если его растворимость в воде при 25 º— равна 1,17·10-3 моль/л.
ƒано:
c 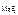 = 1,17Ј10-3 моль/л = 1,17Ј10-3 моль/л
| –ешение –авновесие между осадком малорастворимого электролита и насыщенным раствором над ним выражаетс€ уравнением |
ѕ– 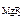 Ц? Ц?
|
MgF2(к) <=> Mg2+ (р) + 2F-(р),
ѕ– 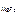 = [Mg2+ ]Ј[F-]2,
= [Mg2+ ]Ј[F-]2,
[Mg2+ ] = c 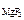 ЈαЈ n
ЈαЈ n 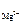 , [F-] = c
, [F-] = c 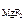 ЈαЈ n
ЈαЈ n 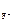 .
.
MgF2 Ц сильный электролит, α = 1;
n 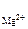 = 1, n
= 1, n 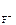 = 2 (из приведенного уравнени€)
= 2 (из приведенного уравнени€)
[Mg2+ ] = 1,17Ј10-3Ј1Ј1 = 1,17Ј10-3 моль/л;
[F-] = 1,17Ј10-3Ј1Ј2 = 2,34Ј10-3 моль/л.
“огда ѕ– 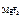 = 1,17Ј10-3Ј(2,34 Ј 10-3)2 = 6,41Ј10-9.
= 1,17Ј10-3Ј(2,34 Ј 10-3)2 = 6,41Ј10-9.
ќтвет: ѕ–  = 6,41Ј10-9.
= 6,41Ј10-9.
8. √»ƒ–ќЋ»« —ќЋ≈…
”–ќ¬≈Ќ№ B
1.Ќаписать ионно-молекул€рные и молекул€рные уравнени€ гидролиза солей:
ј) сульфата хрома (III),
Ѕ) сульфида натри€
» указать реакцию среды их водных растворов.
| ƒано: а) сульфат хрома (III) б) сульфид натри€ | –ешение а) Cr2(SO4)3 диссоциирует в водном растворе: Cr2(SO4)3 → 2Cr3+ + 3SO42- |
| Ќаписать молекул€рные и ионно-молекул€рные уравнени€ гидролиза солей |
ѕод формулой соли написать формулы основани€ и кислоты, которыми образована соль, и подчеркнуть общий ион в формуле соли и слабого электролита.
Cr2 (SO4)3
Cr (OH)3 + H2SO4
—лаб. сильн.
2.Ќаписать сокращенное ионно-молекул€рное уравнение гидролиза с участием одного подчеркнутого иона и одной молекулы воды:
Cr 3+ + H+ OH - <=> + pH < 7, среда кисла€.
3. ѕо полученному сокращенному ионно-молекул€рному уравнению написать полное молекул€рное уравнение. ƒл€ этого каждый ион в сокращенном ионно-молекул€рном уравнении дополнить противоположно зар€женными ионами соли, которые не участвовали в гидролизе. Ќаписать формулы образующихс€ веществ, использу€ правило электронейтральности их молекул, и расставить коэффициенты:
|
|
|
Cr2(SO4)3 + 2H2O <=> 2CrOHSO4 + H2SO4.
б) »спользу€ последовательность написани€ процесса гидролиза, изложенное в а), составл€ем сокращенное ионно-молекул€рное и молекул€рное уравнени€ гидролиза соли Na2S.
Na2S диссоциирует в водном растворе:
Na2S → 2Na+ + S2-.
Na2 S
NaOH + H2 S
—ильн. слаб.






