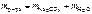 .
.
“огда
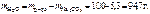
—ледовательно,
 ,
,
где 1000 Ц коэффициент пересчета граммов в килограммы.
ќтвет:  моль/кг.
моль/кг.
5. ќпределить титр раствора вещества, если в 200 см3 раствора содержитс€ 0,1 моль гидроксида кали€.
ƒано:
V р-ра = 200 cм3

| –ешение
“итр раствора вещества определ€ем по формуле
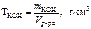 . .
|
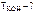
|
—огласно условию задачи  = 0,1 моль,
= 0,1 моль,
откуда m KOH = 0,1Ј M KOH = 0,1Ј56 = 5,6 г,
где ћ ќЌ Ц мол€рна€ масса гидроксида кали€:
ћ ќЌ = 39 + 16 + 1 = 56 г/моль.
—ледовательно, 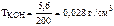 .
.
ќтвет:  = 0,028 г/см3.
= 0,028 г/см3.
6. ќпределить мол€рную долю растворенного вещества в 3,42 %-м растворе сахарозы (—12Ќ22ќ11).
ƒано:

| –ешение ћол€рную долю сахарозы в растворе определ€ем по формуле |
  Ц? Ц?
|

 =
= 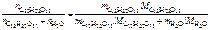 ,
,
где m  и m
и m  Ц соответственно массы сахарозы и воды;
Ц соответственно массы сахарозы и воды;
ћ  и ћ
и ћ  Ц соответственно мол€рные массы сахарозы и воды (342 г/моль и 18 г/моль).
Ц соответственно мол€рные массы сахарозы и воды (342 г/моль и 18 г/моль).
ћассы сахарозы и воды определ€ем согласно условию задачи (3,42 %-й раствор сахарозы).
—ледовательно, в 100 г раствора содержитс€ 3,42 г —12Ќ22ќ11,
m 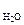 = 100 Ц 3,42 = 96,58 г.
= 100 Ц 3,42 = 96,58 г.

 =
=  .
.
ќтвет: 
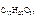 = 0,00186.
= 0,00186.
ЁЌ≈–√≈“» ј ’»ћ»„≈— »’ –≈ј ÷»…
”–ќ¬≈Ќ№ ¬
–ассчитать изменение стандартных энтальпии и энтропии химической реакции
4HCl(г) + O2(г) = 2Ќ2ќ(г) + 2—l2(г)
| ƒано: ”равнени€ химической реакции | –ешение »зменение стандартных энтальпии и энтропии химической реакции рассчитываем на основании первого следстви€ из закона √есса: | |||
| D r Ho(298 K) Ц? D rS o(298 K) Ц? | ||||
D rH o(298 K) = [2D f Ho(298 K, Ќ2ќ(г)) + 2D f Ho(298 K, Cl2(г))] Ц
Ц [4D fH o(298 K, ЌCl(г)) + D fH o(298 K, ќ2(г))];
D rS o(298 K) = [2 S o(298 K, Ќ2ќ(г)) + 2 S o(298 K, Cl2(г))] Ц
Ц [4 S o(298 K, ЌCl(г)) + S o(298 K, ќ2(г))],
где D rH o(298 K, ¬) и S o(298 K, ¬) Ц стандартные энтальпии образовани€ и энтропии веществ, которые находим из таблицы:
| 4HCl(г) | + ќ2(г) | = 2Ќ2ќ(г) | + 2—l2(г) | |
| D rH o(298K) кƒж/моль | 4(-92,3) | 2(-241,84) | ||
| S o(298 K), ƒж/(моль ) | 4(186,8) | 2(188,7) | 2(222,9), |
тогда:
D rH o(298 K) = 2(Ц241,84) Ц 4(Ц92,3) = Ц114,48 кƒж;
∆ rS o(298 K) = (2Ј188,7 + 2Ј222,9) Ц (4Ј186,8 + 205) = Ц129 ƒж/ .
ќтвет: D rH o(298 K) = Ц114,48 кƒж, D rS o(298 K) = Ц129 ƒж/ .
2. —тандартна€ энтальпи€ сгорани€ этилена (—2Ќ4) равна
Ц1410,8 кƒж/моль. Ќаписать термохимическое уравнение сгорани€ этилена и вычислить стандартную энтальпию его образовани€.
| ƒано: D flH o(298 K, C2H4(г)) = = Ц1410,8 кƒж/моль | –ешение —оставл€ем реакцию сгорани€ 1 моль этилена: |
| D fH o(298 K, C2H4(г)) Ц? |
1C2H4(г) + 3ќ2(г) = 2—ќ2(г) + 2Ќ2ќ(ж);
D flH o(298 K) = Ц 1410,8 кƒж/моль.
—тандартной энтальпией сгорани€ вещества называетс€ тепловой эффект реакции полного сгорани€ 1 моль органического вещества до —ќ2(г) и Ќ2ќ(ж) при стандартных услови€х.
—ледовательно, дл€ данной реакции
D flH o(298 K) = D rH o(298 K) = Ц1410,8 кƒж.
ƒл€ определени€ D fH o(298 K, C2H4(г)) используем первое следствие из закона √есса дл€ этой же реакции:
|
|
|
D rH o(298 K) = 2D fH o(298 K, —ќ2(г)) + 2D fH o(298K, Ќ2ќ(ж)) Ц
Ц D fH o(298 K, —2Ќ4(г)) Ц 3D fH o(298 K, ќ2(г)).
ќткуда
D fH o(298 K, —2Ќ4(г)) = 2D fH o(298 K, —ќ2(г)) +
+ 2D fH o(298K, Ќ2ќ(ж)) Ц D rH o(298K) Ц 3D fH o(298K, ќ2(г)).
«начени€ стандартных энтальпий образовани€ (Ќ2ќ(ж) и —ќ2(г)) берем из таблицы:
D fH o(298 K, —2Ќ4(г)) = 2(Ц393,5) +
+ 2(Ц285,8) Ц (Ц1410,8) = 52,2 кƒж/моль.
ќтвет: D fH o(298 K, —2Ќ4(г)) = 52,2 кƒж/моль.
3. ѕо заданным термохимическим уравнени€м рассчитать стандартную энтальпию образовани€ Fe2O3(к) из простых веществ:
Fe(к) +1/2O2(г) = FeO(к), D rH o(298 K, 1) = Ц 264,8 кƒж. (4.1)
4FeO(к) + O2(г) = 2Fe2O3(к), D rH o(298 K, 2) = Ц 585,2 кƒж. (4.2)
| ƒано: “ермохимические уравнени€ двух реакций | –ешение —тандартна€ энтальпи€ образовани€ вещества есть тепловой эффект реакции образовани€ 1 моль Fe2O3(к)из простых веществ при стандартныхуслови€х. «апишем |
| D rH o(298 K) искомой реакции |
термохимическое уравнение реакции образовани€ Fe2O3(к) из простых веществ Fe(к) и O2(г):
2Fe(к) + 3/2O2(г) = Fe2O3(к). (4.3)
»з двух реакций (4.1) и (4.2) необходимо получить реакцию (4.3). ƒл€ этого складываем термохимические уравнени€ (4.1) и (4.2), предварительно умножив на 2 стехиометрические коэффициенты реакции (4.1) и разделив на 2 стехиометрические коэффициенты реакции (4.2):
2Fe(к) + O2(г) + 2FeO(к) + ½O2(г) = 2FeO(к) + Fe2O3(к).
ѕосле сокращени€ получаем уравнение реакции (4.3):
2Fe(к) + 3/2O2(г) = Fe2O3(к).
ƒл€ определени€ изменени€ стандартной энтальпии реакции (4.3) аналогичные математические действи€ осуществл€ем с тепловыми эффектами приведенных реакций (4.1) и (4.2):
D rH o(298 K, 3) = 2D rH o(298 K, 1) + ½D rH o(298 K, 2) =
= 2(-264,8) + ½(-585,2) = Ц 822,2 кƒж.
“ак как реакци€ (4.3) соответствует образованию 1 моль Fe2O3 из простых веществ, то
D fH o(298 K, Fe2O3) = Ц 822,2 кƒж/моль.
ќтвет: D fH o(298 K, Fe2O3) = Ц 822,2 кƒж/моль.
ƒано:
≈ ак = 60 кƒж/моль а) “ 1 = 320 ,
“ 2 = 360 б) “ = 298 ,
 = 48кƒж/моль = 48кƒж/моль
| –ешение
а) »з уравнени€ јррениуса получаем
 = =  ; ;
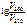 = = 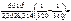 1,09,
где 103 Ц коэффициент пересчета килоджоулей в
джоули, тогда 1,09,
где 103 Ц коэффициент пересчета килоджоулей в
джоули, тогда
 =101,09 = 12,3 раза. =101,09 = 12,3 раза.
|
а)  Ц?
б) Ц?
б) 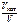 Ц? Ц?
|
| ƒано: ”равнение реакции, р = 1 | –ешение
CO2(г) + C(к) <=> 2CO(г),  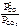 . .
|
| “ Ц? |






