KtA ЂKt+ + AЦ
Kt+ + AЦ + ЌќЌ ЂKtOH + HA
г = [KtOH] [HA] / [Kt+] [AЦ]
KtOH ЂKt+ + OHЦ
[KtOH] [HA] / [Kt+] [AЦ]
b =  Þ [KtOH] =
Þ [KtOH] = 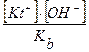
Ќј ЂЌ+ + јЦ а = 

г = [KtOH] [HA] / [Kt+] [AЦ] =  =
= 
ак следует из полученного уравнени€, константа гидролиза не зависит от концентрации соли, образованной слабым основанием и слабой кислотой, а зависит от природы катиона и аниона Ka и Kb и от температуры, т.к. Ka и Kb от нее завис€т.
Kt+ + AЦ + ЌќЌ ЂKtOH + HA
—(1-h) —(1-h) Ch Ch
г =  =
= 
—оли слабых кислот и оснований значительно гидролизованы, поэтому в знаменателе величиной h пренебрегать нельз€.
 =
= 

¬ыведем аналитическую зависимость от рЌ среды дл€ константы гидролиза
г = [KtOH] [HA] / [Kt+] [AЦ]
[KtOH]=[HA], а [Kt+] = [AЦ] = —соли
г = [HA]2 / —соли 2
[HA] =  =
= 
г =  =
= 
 =
= 
[H+]2 = 

pH = Цlg [Ќ+]= Цlg  = 7 Ц½ lgKa + ½ lgKb =7 + ½ рKa Ц ½ pKb
= 7 Ц½ lgKa + ½ lgKb =7 + ½ рKa Ц ½ pKb
—тупенчата€ диссоциаци€ многоосновных кислот и многокислотных оснований. –асчет равновесий концентрации исходных веществ и продуктов электролитической диссоциации различных ступеней.
—“”ѕ≈Ќ„ј“јя ЁЋ≈ “–ќЋ»“»„≈— јя ƒ»——ќ÷»ј÷»я
ћногоосновниые кислоты и многокислотные основани€ диссоциируют ступенчато. ƒл€ кислот число возможных ступеней их диссоциации равно их основности, дл€ оснований Ц кислотности. »х свойства особенно под вли€нием природы растворител€ могут существенно различатьс€.
онстанта диссоциации предшествующей ступени всегда много больше таковой последующей ступени за счет того, что одноименные ионы продуктов реакции предшествующей ступени смещают равновесие электролитической диссоциации в стороны недиссоциированной формы (принцип Ће-Ўателье).
Ќ3–ќ4 ЂЌ+ +Ќ2–ќ4Ц 

Ќ2–ќ4- ЂЌ+ +Ќ–ќ42Ц 

Ќ–ќ42- ЂЌ+ +–ќ43Ц 

ƒиссоциаци€ по первой ступени всегда больше, чем по следующим, т.к. от нейтральной частицы катиону водорода оторватьс€ легче, чем от зар€женного аниона.
Ќаличие последовательных ступеней электролитической диссоциации приводит к тому, что в растворе всегда имеютс€ в соизмеримом количестве два типа анионов. (дл€ 3-х и более основных кислотах). 3-им типом анионов можно пренебречь в силу малой его концентрации.
Ќ3–ќ4 Ќ2–ќ4- Ќ–ќ42- –ќ43-
онцентраци€ кислоты в растворе складываетс€ из равновесных концентраций [Ќ3–ќ4] + [Ќ2–ќ4-] + [Ќ–ќ42-] + [–ќ43-] = C (Ќ3–ќ4)
Ќайдем долю каждой частицы в растворе ri




 =
= 

 =
= 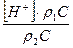

 =
= 

Ќ3–ќ4 ЂЌ+ +Ќ2–ќ4Ц
C-Ca Ca
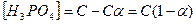

 уравнение јррениуса
уравнение јррениуса
его можно преобразовать к виду 
¬ данном случае следует использовать константу диссоциации по 1-й ступени. ќдин из корней этого уравнение отбрасывают (отрицательное значение или значение больше нул€), другое используют a1 или a2.
 (по первой ступени считаем)
(по первой ступени считаем)


»меетс€ слаба€ одноосновна€ кислота Ќј ЂЌ+ + ј-
Ќайти зависимость концентрации анионов и недиссоциированной формы от исходной концентрации кислоты
|
|
|


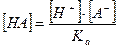 , тогда
, тогда
 =
=  + [A-] = [A-]
+ [A-] = [A-] 
[A-] = CHA/  =
=  ;
;  =aC
=aC
ћожно найти долю частиц  дол€ анионов ј- в общей концентрации всех частиц.
дол€ анионов ј- в общей концентрации всех частиц.
«ависимость концентрации недиссоциированной формы от концентрации кислоты Ќј —Ќј

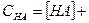
 =
= 

ƒол€ частиц 






