1.–еакци€ с катионами бари€ (фармакопейна€).
¬а2+ + SO42- → BaSO4↓ (белый мелкокристаллический)
¬ыпадает белый осадок сульфата бари€. ≈сли в растворе присутствует перманганат кали€ ћќ4, то осадок BaSO4 окрашиваетс€ в фиолетово - красный цвет за счет адсорбции ћпќ42+ - ионов на осадке.
BaSO4 не раствор€етс€ в минеральных кислотах, за исключением концентрированной H2SO4, в которой он частично растворим с образованием Ba(HSO4)2:
BaSO4 + H2SO4→Ba(HSO4)2
2.–еакци€ с катионами свинца.
–№2+ + SO42- →PbSO4↓. (белый кристаллический)
ќсадок PbSO4 частично раствор€етс€ в минеральных кислотах; раствор€етс€ в щелочах и в водных растворах ацетатов натри€ CH3COONa или аммони€ CH3COONH4 с образованием комплексных соединений:
PbSO4 + 4NaOH →Na2[Pb(OH)4] + Na2SO4
3. –еакци€ с родизонатом бари€.
Ќа листок фильтровальной бумаги нанос€т раствор ¬а—12 и родизоната натри€ или родизоновый кислоты. Ќа бумаге возникает красное п€тно родизоната бари€. Ќа это п€тно нанос€т 1-2 капли раствора, содержащего сульфат - ионы (Na2SO4, K2SO4 или H2SO4 разбавленной). ѕ€тно обесцвечиваетс€.
6. ѕриведите уравнени€ реакций идентификации кали€ сульфита. ”кажите аналитические эффекты реакций, особенности их выполнени€. каким аналитическим группам относ€тс€ катион и анион, вход€щие в состав соли? ”кажите групповой реагент, аналитический эффект при действии группового реагента.
али€ сульфит
+ - 1 анал.гр.катионов, групп. реагента нет
SO32- - 1 анал.гр.анионов,групп.реагент соли бари€
1.–еакци€ с гексанитрокобальтатом натри€ (желт.↓)
K2SO3 +Na3[Co(No2)6]→K2 Na[Co(No2)6]↓ +2NaCl
2.–еакци€ с Na гидротартратом (бел.крист.↓)
K2SO3 +NaHC4H4O6→K HC4H4O6↓+NaCl
3.K+→плам€ фиолет.цвет
4. — сол€ми бари€ (бел.крист.↓)
K2SO3+BaCl2→Ba SO3↓+2NaCl
5.–азложение кислотами(характер.запах)
K2SO3+2HCl→2KCl+H2O+SO2↑
6.— раствором йода (‘)-обесцв.раствора
K2SO3+I2+H2O→ K2SO4+2KI
ѕриведите уравнени€ реакций идентификации натри€ арсенита. ”кажите аналитические эффекты реакций, особенности их выполнени€. каким аналитическим группам относ€тс€ катион и анион, вход€щие в состав соли? ”кажите групповой реагент, аналитический эффект при действии группового реагента.
Ќатри€ арсенит
Na+ -1 анал.гр.катионов, групп. реагента нет
AsO3- -1 анал.гр.анионов,групп.реагент соли бари€
1.— цинкуранилацетатом (под микрскопом желт тетраэдрич. и октаэдр.кристалы)
NaAsO3+ Zn(UO2)3(CH3COO)8+CH3COOH→Na Zn(UO2)3(CH3COO)9+HAsO3
2. с гексагидроксостибатом кали€ (под микрос.белые кристаллы)
NaAsO3+K[Sb(OH)6]→ Na[Sb(OH)6]↓+ K AsO3
3.Na+→плам€ желт.цвет
4. — сол€ми бари€ (бел.крист.↓)
2 NaAsO3 +3BaCl2→Ba3(AsO3)2↓+ 6NaCl
5.–еакции с сульфид ионами в кислой среде (ф) Цжелт.осадок
6. C нитратом серебра (ф)
NaAsO3 + AgNO3→NaNO3+AgAsO3↓
ѕриведите уравнени€ реакций идентификации аммони€ сульфида. ”кажите аналитические эффекты реакций, особенности их выполнени€. каким аналитическим группам относ€тс€ катион и анион, вход€щие в состав соли? ”кажите групповой реагент, аналитический эффект при действии группового реагента.
|
|
|
јммони€ сульфид
NH4+- 1 анал.гр.катионов, групп. реагента нет
S-2- 2 аналит.группа анионов.√руппов.реагент AgNO3
1. ѕары аммиака окрашив.красн.лакмус.бумагу в синий цвет
(NH4)2S +2NaOH→NH3↑+H2O+Na2S
2. — раствором Ќесслера(осад.красно-бур.аморфный)
(NH4)2S +2K2[HgI4]+4KOH→[NH2Hg2O]I↓+7KI+HCl+ H2O
3. C нитратом серебра(черный осадок)
(NH4)2S + AgNO3→Ag2S↓+2NH4NO3
4.— сильными кислотами(характер.запах сероводорода,фильтров.бумага пропитанна€ ацетатом свинца чернеет)
(NH4)2S +2HCl→H2S↑+2NH4Cl
ѕриведите уравнени€ реакций идентификации аммони€ роданида. ”кажите аналитические эффекты реакций, особенности их выполнени€. каким аналитическим группам относ€тс€ катион и анион, вход€щие в состав соли? ”кажите групповой реагент, аналитический эффект при действии группового реагента.
јммони€ роданид
NH4+- 1 анал.гр.катионов, групп. реагента нет
SCN--2 аналит.группа анионов.√руппов.реагент AgNO3
1. –-и€ с щелочами пары аммиака окрашив.красн.лакмус.бумагу в синий цвет
NH4 SCN+NaOH→ NH3↑+H2O+NaSCN
2.— раствором несслера (осад.красно-бур.аморфный)
NH4 SCN+2K2[HgI4]+4KOH→[NH2Hg2O]I↓+7KI+HCl+ 3H2O
3. C нитратом серебра(белый ос)
NH4 SCN+ AgNO3→Ag SCN↓+ NH4 NO3
4.— сол€ми кобальта и 5-6к.диэтилов.эфира Цвстр€хив.и верхний органич.слой окрашиваетс€ в синий цв.
4NH4 SCN+—oCl2→ (NH4)2[Co(SCN)4]+2NH4Cl
ѕриведите уравнени€ реакций идентификации висмута нитрата. ”кажите аналитические эффекты реакций, особенности их выполнени€. каким аналитическим группам относ€тс€ катион и анион, вход€щие в состав соли? ”кажите групповой реагент, аналитический эффект при действии группового реагента.
¬исмут-катион 5 аналитической группы. √рупповой реагент группы €вл€ютс€ щелочи или гидроксид аммони€, получаютс€ гидроксиды металлов, нерастворимые в избытке реагента.
1. –еакци€ с щелочами и аммиаком: (Bi—16)3- + 3 ќЌ- = Bi (ќЌ) 3 (белый) + 6 —1-
ќсадок расвор€етс€ в минеральных кислотах. ѕри нагревании желтеет вследствие образовани€ оксогидроксида висмута (III): Bi (ќЌ) 3 = Bi ќЌ + Ќ2ќ
–еакци€ гидролиза: (Bi—16)3- + Ќ2ќ = Biќ—l (белый) + 2 Ќ—l + 3 —l-
ќсадок не раствор€етс€ в растворах винной кислоты и ее солей.
3. –еакци€ с сульфид-ионами (фармакопейна€)
(Bi—l6)3- + 3 S2- = Bi2 S3(черно-коричневый) + 12 —l-
в кислой среде. ќсадок не раствор€етс€ в разбавленных минеральных кислотах, за исключением разбавленной HNO3, в которой он раствор€етс€ с выделением свободной серы:
Bi2S3 + 8 HNO3 = 2 Bi (NO3)3 + 2 NO + 2S + 4 H20
ќсадок Bi2S3 раствор€етс€ в присутствии хлорида железа (III) FeCl3: Bi2S3+6 FeCl3 --> 2 BiCl3 + 6 FeCl2+ 3S
4.–еакци€ с иодидами (фармакопейна€).
(Bi—16)3- + 3 I- = Bi I3 (черный) + 6 —l-
ƒальнейшее прибавление избытка раствора KI приводит к растворению осадка и образованию оранжевого раствора:
Bil3 + I- = [Bil4]- (желто-оранжевый)
ѕри прибавлении воды к этому раствору и его нагревании сначала выпадает осадок BiI3, который затем гидролизуетс€ с образованием желто-оранжевого осадка оксоиодида висмута BiOI (иодида висмутила):
|
|
|
[Bil4]- + Ќ2ќ = BiOI + 3 I- + 2Ќ+.
5.–еакци€ восстановлени€ висмута (III) до висмута (ќ) соединени€ми олова (II).
2 Bi(OH)3 + 3 [Sn(OH)4]2- = 2 Bi∞ +3 [Sn(OH)6]2-, pЌ=10.
јналитические реакции нитрат - иона NO3-
ќтноситс€к 3-й аналитической группе.√руппового реагента нет.
1.–еакци€ с дифениламином (фармакопейна€).
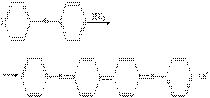
2.–еакци€ с металлической медью (фармакопейна€).
–еакцию провод€т в среде концентрированной H2SO4 при нагревании.
2KNO3 + 2H2SO4 + Cu --> K2SO4 + 2NO2 + CuSO4 + 2H2O,жeлтo-бypыe пары
3.–еакци€ с сульфатом железа (II) и концентрированной серной кислотой.
–еакцию провод€т при нагревании:
3Fe2+ + NO3- + 4H+ = 3Fe3+ + NO + 2H2O,
Fe2+ + NO = [FeNO]2+.(бурый)
Ёту реакцию дает и нитрит - ион, поэтому нельз€ открыть этой реакцией нитрат - ион в присутствии нитрит- иона. ѕроведению реакции мешают ¬г-, √, SO32-, S2O32', CrO42-, MnO42-.
4.–еакци€ с металлическим алюминием или цинком.
3 NO3- + 8 ј1 + 5 ќЌ- + 18 Ќ2ќ -> 3 NH3 + 8 [Al (OH)4]-
¬ыдел€ющийс€ аммиак ощущаетс€ по запаху и окрашивает влажную красную лакмусовую бумагу в синий цвет.ѕроведению реакции мешают катионы аммони€ NH4+, выдел€ющие аммиак в щелочной среде, а также другие анионы, способные восстанавливатьс€ до аммиака (NO2-, SCN-, ферро - и феррицианид -- ионы).
5.–еакци€ с антипирином. –еакцию провод€т в кислой среде.
ѕриведите уравнени€ реакций идентификации магни€ сульфата. ”кажите аналитические эффекты реакций, особенности их выполнени€. каким аналитическим группам относ€тс€ катион и анион, вход€щие в состав соли? ”кажите групповой реагент, аналитический эффект при действии группового реагента.
јналитические реакции катионов магни€ Mg 2+
V аналитической группы
√рупповым реагентом на катионы V аналитической группы €вл€ютс€ щелочи или гидроксид аммони€, получаютс€ гидроксиды металлов, нерастворимые в избытке реагента.
1. –еакци€ с щелочами и аммиаком: Mg2+ + 2 ќЌ- --> Mg(OH)2 (белый аморфный)
Mg2+ + 2 NH4 OH= Mg(OH)2 + 2 NH4+
ќсадок не раствор€етс€ в щелочах, раствор€етс€ в Ќ—l, H2SO4, CH3COOH: Mg(OH)2 + 2 Ќ+ -> Mg2+ + 2 Ќ2ќ
2. –еакци€ с гидрофосфатом натри€ Na2HPO4 (фармакопейна€).
–еакцию провод€т в аммиачном буфере:
Mg2+ + HPO42- + NH4OH = MgNH4PO4↓ + H2O. (белый кристаллический) ѕри проведении реакции в отсутствии катионов аммони€ и аммиака выпадает белый аморфный осадок MgHPO4. »збыток NH4 мешает выпадению NH4MgPO4. ќсадок NH4MgPO4 раствор€етс€ в минеральных кислотах и в уксусной кислоте:
NH4MgPO4 + 3 Ќ—1 - Ќ3–ќ4 + MgCl2 + NH4C1
NH4MgPO4+ 2 CHjCOOH = NH4H2PO4 + (CH3COO)2Mg
ѕроведению реакции мешают катионы Li+, Ca2+, Sr2, Ba2+ и др.
3. –еакци€ с магнезоном I - п - нитробензолазорезорцином.
¬ щелочной среде магнезон I, имеющий красную окраску, образует с катионами ћg2+,комплекс синего цвета, сорбирующийс€ на осадке Mg(OH)2:
ѕри малых концентраци€х Mg2+ осадок не выдел€етс€, а раствор окрашиваетс€ в синий цвет. ѕроведению реакции мешают Cd2+, Sn2+, Cr3+, Fe2+, Co2+, Ni2+.
4.–еакци€ с 8- оксихинолином.
–еакцию провод€т в аммиачной среде при рЌ = 8--13 (при нагревании).
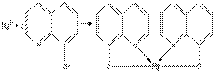
ќсадок растворим в минеральных кислотах и в уксусной кислоте. ѕроведению реакции мешают катионы, также образующие комплексы с 8 -оксихинолином (Cu2+, Zn2+. Cd2+, Fe3+ и др.).
5. –еакци€ с хинализарином.
–еакцию провод€т в щелочной среде. ќбразуетс€ синий осадок комплексного соединени€ - хинализарината магни€ состава MgL(OH), где ЌL - условное обозначение молекулы хинализарина:
ѕри небольшом содержании катионов Mg2+в растворе осадок не выпадает, а раствор окрашиваетс€ в цвет. ѕроведению реакций мешают катионы алюмини€.
6. –еакци€ с растворимыми карбонатами (Nа2—03).
2 Mg2++ 2 Nа2—03 + Ќ2ќ = (ћgќЌ)2—0з (белый аморфный) +4 Nа + —ќ2. ќсадок (ћgќЌ)2—0з,растворим в кислотах и в сол€х аммони€.
7. –еакци€ с оксалатом аммони€ (NH4)—2ќ4.
Mg2++ (NH4)—2ќ4 = Mg —2ќ4 (белый) + 2 NH4
8. атионы Mg2+ с дифенилкарбазидом (—6Ќ5 NHNH)2—0 образуют комплекс красно-- фиолетового цвета.
|
|
|
јналитические реакции сульфат-иона SO42-
јнион первой аналитической группы,групповой реагент - хлорид бари€ ¬а—l2 в нейтральном или слабо≠щелочном растворе
1.–еакци€ с катионами бари€ (фармакопейна€).
¬а2+ + SO42- --> BaSO4 (белый мелкокристаллический)
BaSO4 не раствор€етс€ в минеральных кислотах, за исключением концентрированной H2SO4, в которой он частично растворим с образованием Ba(HSO4)2:
BaSO4 + H2SO4->Ba(HSO4)2
2.–еакци€ с катионами свинца.
–№2т + SO42- -> PbSO4. (белый кристаллический)
ќсадок PbSO4 частично раствор€етс€ в минеральных кислотах; раствор€етс€ в щелочах и в водных растворах ацетатов натри€ CH3COONa или аммони€ CH3COONH4 с образованием комплексных соединений:
PbSO4 + 4 NaOH -- Na2[Pb(OH)4] + Na2SO4
3. –еакци€ с родизонатом бари€.
Ќа листок фильтровальной бумаги нанос€т каплю раствора ¬а—12 и 1 каплю раствора родизоната натри€ Na2C6Oe или родизоновый кислоты Ќ2—б06. Ќа бумаге возникает красное п€тно родизоната бари€. Ќа это п€тно нанос€т 1-2 капли раствора, содержащего сульфат - ионы (Na2SO4, K2~SO4 или H2SO4 разбавленной). ѕ€тно обесцвечиваетс€.
ѕриведите уравнени€ реакций идентификации ацетата ртути (I). ”кажите аналитические эффекты реакций, особенности их выполнени€. каким аналитическим группам относ€тс€ катион и анион, вход€щие в состав соли? ”кажите групповой реагент, аналитический эффект при действии группового реагента.
Hg22+
¬тора€ группа катионов.
1.√рупповой реагент Ц сол€на€ кислота.
Hg22+ + 2Cl- = Hg2Cl2↓.
’лорид ртути взаимодействует с гидроксидом аммони€, образу€ хлорид меркураммони€
[Hg(NH2)]Cl, и металлическую ртуть, вследствие чего осадок чернеет:
Hg2Cl2 + 2NH4OH = [Hg(NH2)]Cl↓ + Hg↓ + NH4Cl + 2H2O.
2. атион ртути способен восстанавливатьс€ в присутствии восстановителей (Sn2+, Cu) до металлической ртути:
Hg22+ + Sn2+ = 2Hg↓ + Sn4+;
Hg22+ + Cu = 2Hg↓ + Cu2+.
ќбразуетс€ осадок черного цвета.
3. –еакци€ с сол€ми йодистоводородной кислоты:
Hg22+ + 2I- = Hg2I2↓.
ќбразуетс€ гр€зно-зеленый осадок йодида ртути (I), растворимый в избытке реагента с образованием тетраиодо(II)меркурата кали€ и черного осадка металлической ртути:
Hg2I2 + 2KI = K2[HgI4] + Hg↓.
4. –еакци€ со щелочами:
Hg22+ + 2OH- = Hg2O↓ + H2O.
¬ результате реакции образуетс€ неустойчивый гидроксид ртути (I), разлагающийс€ на оксид ртути (I) черного цвета и воду.
5. –еакци€ с хроматом кали€:
Hg22+ + CrO42- = Hg2CrO4v.
ќбразуетс€ кирпично-красный осадок хромата ртути, растворимый в азотной кислоте.
ацетат - ион CH3COO'
ќбщего группового реагента нет. јнионы III группы
1.–еакци€ с хлоридом железа (III) (фармакопейна€).
ѕри рЌ = 5-8 ацетат - ион с катионами Fe(III) образует растворимый темно - красный (цвета крепкого.ча€) ацетат или оксиацетат железа (III).
¬ водном растворе он частично гидролизуетс€; подкисление раствора минеральными кислотами подавл€ет гидролиз и приводит к исчезновению красной окраски раствора.
3 —Ќз—ќќЌ + Fe -->* (CH3COO)3Fe + 3 Ќ+
ѕри кип€чении из раствора выпадает красно-бурый осадок основного ацетата железа (III):
(CH3COO)3Fe + 2 Ќ2ќ <- Fe(OH)2 CH3COO + 2 —Ќ3—ќќЌ
2.–еакци€ с серной кислотой.
јцетат - ион в сильно кислой среде переходит в слабую уксусную кислоту, пары которой имеют характерный запах уксуса:
CH3COO- + H+ =CH3COOH.
3.–еакци€ образовани€ уксусноэтилового эфира (фармакопейна€).
CH3COO- + H+ =CH3COOH,
CH3COOH + C2H5OH → CH3COOC2H5 + H2O.
¬ кислой среде выдел€етс€ уксусна€ кислота, котора€ со спиртами образует летучие эфиры, обладающие характерным запахом.
|
|
|
ѕриведите уравнени€ реакций идентификации сульфида стронци€. ”кажите аналитические эффекты реакций, особенности их выполнени€. каким аналитическим группам относ€тс€ катион и анион, вход€щие в состав соли? ”кажите групповой реагент, аналитический эффект при действии группового реагента.
Sr2+-“реть€ группа катионов. √рупповой реагент - разбавленна€ серна€ кислота и ее соли, образующие с катионами данной группы малорастворимые сульфаты, нерастворимые в воде, кислотах и щелочах:
1. Sr2+ + SO42- = SrSO4↓.
2. ќкрашивание пламени Ћетучие соли стронци€ окрашивают плам€ горелки в карминово-красный цвет.
3. арбонат аммони€ (NH4)2CO3 ѕри взаимодействии с растворами солей стронци€ осаждает карбонат стронци€ белого цвета, растворимый в уксусной, сол€ной и азотной кислотах:
SrCl2 + (NH4)2CO3 = SrCO3+ 2NH4Cl
4. Ќасыщенный раствор гипса CaSO4 . 2H2O (гипсова€ вода) образует с ионами Sr2+ белый осадок сульфата стронци€:
Sr2+ + SO42- = SrSO4
—ульфид-ион:втора€ аналитическа€ группа. √рупповой реагент - Ќитрат серебра AgNO3 и присутствии HNO3
1. 2Ag+ + S2- = Ag2S↓ „ерный
2. –еакци€ с кислотами
Na2S + H2SO4 = H2S + Na2SO4.
¬ыделение сероводорода легко обнаружить по запаху или по почернению бумажки, смоченной раствором ацетата свинца:
Pb(CH3COOH)2 + H2S = PbS + 2CH3COOH.
3. –еакци€ с сол€ми кадми€
Na2S + Cd(NO3)2 = CdS + 2NaNO3.
ѕри добавлении к раствору, содержащему сульфид-ион, солей кадми€ образуетс€ желтый осадок сульфида кадми€.
4. –еакци€ с нитрозопентациано(II)ферратом натри€ (нитропруссидом натри€)
Na2[Fe(CN)5NO] + Na2S = Na4[Fe(CN)5NOS].
–аствор окрашиваетс€ в красно-фиолетовый цвет вследствие образовани€ комплекса
5. –еакци€ с нитратом серебра:
S2- + 2Ag+ = Ag2S↓.
Ќитрат серебра образует с сульфид-ионом черный осадок сульфида серебра, нерастворимый в NH4OH, Na2S2O3 и KCN, но растворимый при нагревании в 2 ћ HNO3. ћешающих обнаружению анионов нет.
6. –еакци€ с гидроксокомплексом свинца Pb(OH)42-:
Pb2+ + 2OH- = Pb(OH)2↓,
Pb(OH)2 + 2OH- = Pb(OH)42-,
S2- + Pb(OH)42- = PbS + 4OH-.
√идроксокомплекс свинца образует с сульфид-ионом черный осадок PbS, растворимый при нагревании в 2 ћ HNO3:
3PbS + 8HNO3 = 3Pb(NO3)2 + 3S + 2NO + 4H2O.
ѕриведите уравнени€ реакций идентификации кали€ фосфата. ”кажите аналитические эффекты реакций, особенности их выполнени€. каким аналитическим группам относ€тс€ катион и анион, вход€щие в состав соли? ”кажите групповой реагент, аналитический эффект при действии группового реагента.
атион кали€
I јналитическа€ группа характеризуетс€ отсутствием группового реагента
1. –еакци€ с гидротартратом натри€ или (винной кислотой) в присутствии ацетата натри€ (рЌ=7)
K+ + HC4H4O6- = KHC4H4O6↓.бел. крист.
2. –еакци€ с гексанитрокобальтатом (III) натри€ (рЌлюба€ кроме сильных кислот и сильных щелочей, т.к. реагент разлагаетс€)
2K+ + Na3[Co(NO2)6] = NaK2[Co(NO2)6]↓ + 2Na+. желт, крист.,
–еакци€ с гексанитрокупратом (II) натри€ и свинца.
2 + + Na2Pb[Cu(NO2)6] = K2Pb[Cu(NO,)6] | черн. кубические крист. + 2Na+
4. ќкрашивание пламени газовой горелки - в фиолетовый цвет.
‘осфат-ион
анион I аналитической группы-глорид бари€ BaCl2 или нитрат бари€ Ba(NO3)2. ѕри взаимодействии анионов первой группы с катионом бари€ в растворе образуютс€ осадки соответствующих бариевых солей:
1.–еакци€ с ¬а—12.
2 –ќ43- + 3 ¬а2+ -= ¬а3(–ќ4)2 (белый)
Ќ–ќ42- + ¬а2+ -+ ¬аЌ–ќ4 (белый)
¬ аммиачной среде реакци€ гидрофосфат- ионов с катионами бари€ приводит к образованию осадка среднего ортофосфата бари€ ¬а3(–ќ4)2:
2 Ќ–ќ42- + 3 ¬а2+ + 2 NH3 -> ¬а3(–ќ4)2 + 2 NH4+
—вежеосажденный осадок ¬а3(–ќ4)2 раствор€етс€ в FfNO3, HC1, —Ќ3—ќќЌ.
2.–еакци€ с нитратом серебра (фармакопейна€).
–еакцию провод€т в нейтральной среде:
–ќ43- + 3 Ag+ -> Ag3PO4 (желтый)
ќсадок раствор€етс€ в азотной кислоте, в концентрированном аммиаке.
3.–еакци€ с магнезиальной смесью (фармакопейна€)
√идрофосфат - ион Ќ–ќ42~ при взаимодействии с магнезиальной смесью (MgCI2 + NH4CI + NH3) образует белый мелкокристаллический осадок магнийаммонийфосфата NH4MgPO4:
HPO42- + NH4OH + Mg2+ = MgHPO4↓ + H2O.
4. –еакци€ с молибдатом аммони€ (фармакопейна€). –еакцию провод€т в азотнокислой среде при нагревании:
PO43- + NH4+ + 12MoO42- + 24H+ = (NH4)3P(Mo3O10)4↓ + 12H2O.
¬ыпадает желтый осадок фосфоромолибдата аммони€.
Ёта формула к первому разделу к 10 вопросу к нитритомерии.
Ќитритометрическое определение стрептоцида основано на реакции диазотировани€:
NH2 N ≡N+
 + NaNO2 + Ќ—1 ↔
+ NaNO2 + Ќ—1 ↔ 

 —l - + NaC1+ 2Ќ2ќ
—l - + NaC1+ 2Ќ2ќ
SO3H SO3H
|
|
|






