”слови€ проведени€ опыта
1. ѕри выполнении опыта необходимо брать избыток реактива Ќесслера, так как осадок растворим в сол€х аммони€.
2. ќткрытию катиона NH4+ мешают катионы Fe3+, Cr3+, Co2+, Ni2+ и другие.
3. ¬ присутствии этих катионов ведут, добавл€€ 50% раствор тартрата кали€ NaC4H4O6, который с большинством катионов дает комплексные соединени€.
онтрольна€ задача
“≈ћј: јнализ смеси катионов первой группы
’од анализа:
1. атион NH4+ можно открыть в присутствии катионов + и Na+ реактивом Ќесслера или реакцией с гидроксидом натри€.
ќткрытию катионов + и Na+. ѕоэтому, если мешает катион NH+. ѕоэтому, если в исследуемом растворе обнаружен катион NH4+, перед открытием катионов кали€ и натри€ его следует удалить из раствора. ƒл€ этого возьмите 15-20 капель исследуемого раствора, поместите в тигель или в фарфоровую чашку и выпарьте досуха. ќсадок в тигле прокалите до полного прекращени€ выделени€ белого УдымаФ. ќхладите содержимое тигл€ и обработайте 8-10 капл€ми дистиллированной воды, тщательно перемешайте, после этого проверьте на полноту удалени€ аммони€. “олько после отрицательной реакции на реактив Ќесслера можно приступить к открытию катионов.
2. ќбнаружение катиона +. Ќа 2-3 капли исследуемого раствора подействуйте 3-4 капл€ми раствора гексанитро-(III) кобальтата натри€, дайте посто€ть. ∆елтый осадок гексанитро-(III) кобальтата натри€-кали€ K2Na[Co(NO2)6] укажет на присутствие катиона +.
ѕроведите реакцию на катион + гексанитро-(II) купрата натри€-свинца Na2Pb[Cu(NO2)6] и рассмотрите осадок под микроскопом.
3. атион Na+ обнаруживаетс€ дигидроантимонатом в присутствии катионов +. ¬озьмите 3-4 капли сконцентрированного путем выпаривани€ исследуемого раствора, прибавьте в пробирку такое же количество дигидроантимоната кали€ и потрите о стенки пробирки стекл€нной палочкой. ”бедитесь, что осадок кристаллический. Ќе забудьте соблюдать все услови€ открыти€ катиона Na+.
ƒве капли исследуемого раствора выпарьте на предметном стекле, дайте остыть и обработайте каплей раствора уранилацетата. ѕосле проведени€ работы сделайте вывод о присутствии катионов первой группы в исследуемом растворе.
Ћабораторна€ работа є2
“≈ћј: ¬тора€ аналитическа€ группа катионов (анализ катионов группы хлороводородной кислоты)
√рупповой реагент Ц хлороводородна€ кислота.
атионы Ag+, Pb2+, [Hg2]2+ при взаимодействии с хлороводородной кислотой образуют труднорастворимые в воде и в разбавленных кислотах осадки:
AgNO3 + HCl → AgCl↓ + HNO3
Ag+ + Cl-→ FgCl↓
Pb(NO3)2 + 2HCl → PbCl2↓ + 2HNO3
Pb2+ + 2Cl-→ PbCl2↓
Hg2(NO3)2 + 2HCl → Hg2—l2↓ + 2HNO3
[Hg2]2+ + 2Cl-→ Hg2Cl2↓
Cледует избегать избытка и использовани€ концентрированной хлороводородной кислоты, так как могут образоватьс€ растворимые комплексные соединени€:
AgCl + 2HCl → H2[AgCl3], PbCl2+ HCl→ H[PbCl3]
ѕри температуре воды 100∞ растворимость хлорида свинца увеличиваетс€ в три раза, в то врем€ как растворимость хлорида серебра и хлорида ртути практически остаетс€ прежней. Ёто свойство используетс€ дл€ определени€ катионов Pb2+ от катионов [Hg2]2+ и Ag+.
|
|
|
’лорид ртути при взаимодействии с раствором аммиака образует хлорид димеркураммони€, который неустойчив и разлагаетс€ на малорастворимый меркураммоний и металлическую ртуть, котора€ придает осадку черный цвет:
Hg2Cl2 + 2NH4OH → [Hg2NH2]Cl↓ + NH4Cl + 2H2O
Hg2Cl2 + 2NH4OH → [Hg2NH2]Cl↓ + Cl- + 2H2O
[Hg2NH2]Cl→ [NH2Hg]Cl + Hg↓
Ёто позвол€ет отдел€ть катион[Hg2]2+ от катиона Ag+.
’лорид серебра хорошо растворим под действием аммиака с образованием комплексной соли:
AgCl + 2NH4OH→ [Ag(NH3)2]Cl + 2H2O
AgCl + 2NH4OH→ [Ag(NH3)2]++ Cl-+ 2H2O
„астные реакции катионов Ag+
1. √идроксиды кали€ и натри€ KOH и NaOH образуют с катионом Ag+ бурый осадок оксида серебра Ag2ќ:
AgNO3 + KOH → AgOH↓ + KNO3
Ag+ +OH- → AgOH; AgOH→ Ag2O↓ + H2O
ќксид серебра (I) раствор€етс€ в растворе аммиака NH3:
Ag2O + 4NH4OH → 2[Ag(NH3]2OH + H2O
2. ’ромат кали€ K2CrO4 дает с катионом Ag+ осадок хромата серебра Ag2CrO4 кирпично-красного цвета:
K2CrO4 + 2AgNO3 → Ag2CrO4↓ +2KNO3
CrO42- + 2Ag2+→ Ag2CrO4
ќпыт. ¬озьмите в пробирку 2-3 капли раствора нитрата серебра и добавьте 3-4 капли дистиллированной воды и 1-2 капли хромата кали€. ќбратите внимание на цвет осадка и проверьте его растворимость.
”слови€ проведени€ опыта.
1. –еакцию следует проводить при рЌ= 6,5 Ц 7,5.
2. ¬ аммиачной и сильно кислой среде осадок не образуетс€.
3. »оны Pb2+, Ba2+ и др., образующие с CrO42 - осадки, мешают проведению реакции.
3. Ѕромид и иодид кали€ KBr и KI образуют с катионом Ag+ бледно-желтый осадок бромида серебра AgBr и желтый осадок иодида серебра AgI: KBr + AgNO3 → AgBr↓ + KNO3; Ag+ + Br-→ AgBr↓
KI + AgNO3-→ AgI↓ + KNO3; Ag+ + I-→ AgI↓
„астные реакции катиона Pb2+
1. √идроксиды ќЌ и NaOH образуют с катионом Pb2+ белый осадок Pb(OH)2, растворимый как в кислтах, так и в концентрированных растворах гидроксидов:
Pb(NO3)2 + 2 NaOH→ Pb(OH)2↓ + 2NaNO3
Pb2+ + 2OH-→ Pb(OH)2¯
ѕри действии избытка гидроксида образуетс€ плюмбит натри€:
Pb(OH)2+ 2NaOH → Na2PbO2
2. —ерна€ кислота и сульфаты осаждают катионы Pb2+ выпадает белый осадок PbSO4. ѕри нагревании сульфатов свинца с растворами гидроксидов образуютс€ плюмбиты:
PbSO4 + 4 ќЌ → 2PbO2 + K2SO +2H2O
PbSO4 + 4OH- → PbO22-+ 2H2O
—ульфат свинца раствор€етс€ в 30% растворе ацетата аммони€:
2 PbSO4 + 2—Ќ3—ќќ- → [Pb(CH3COO)2PbSO4] + SO42-
јзотна€ и хлороводородна€ кислоты повышают растворимость сульфата свинца, так как ионы Ќ+ св€зываютс€ ионами SO42- с образованием аниона ЌSO4-: PbSO4 ↔ Pb2+ + SO42-
HNO3 ↔ NO3- +H+; H++ SO4-↔ HSO4 -
3. ’ромат кали€ 2—rO4 и дихромат кали€ K2Cr2O7 образуют с катионом Pb2+ малорастворимый хромат свинца желтого цвета:
Pb2+ + CrO42-→ PbCrO4
’ромат свинца растворим в гидроксидах, но не растворим в уксусной кислоте.
4. »он I- образует с катионом Pb2+ желтый осадок:
Pb2++ 2I- → PbI2↓
ќпыт: ѕолучите осадок иодида свинца PbI2, возьмите часть его и прибавьте несколько капель воды и 2н раствора уксусной кислоты и нагрейте. ќсадок раствор€етс€, но при охлаждении вновь образуетс€ в виде блест€щих золотистых кристаллов.
|
|
|
”слови€ проведени€ опыта:
1. –еакцию провод€т при рЌ= 3-5.
2. ¬ избытке KI осадок PbI2 раствор€етс€, образу€ комплексное соединение K2[PbI4].
3. Ёта реакци€ катионов Pb2+ позвол€ет открыть их в присутствии всех катионов аналитических групп.
5. ƒифенилкарбазон (дитизон)
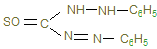
образует с сол€ми свинца внутрикомплексное соединение, окрашенное в кирпично-красный цвет. –еакци€ чувствительна€.
„астные реакции катиона [Hg2]2+
¬се соли ртути €довиты!
1. √идроксиды образуют с катионом [ Hg2]2+ черный осадок оксида ртути(I):
Hg2(NO3)2 + 2NaOH → Hg2O↓ + 2NaNO3 + H2O
[Hg2]2+ + 2OH- → Hg2O + H2O
–аствор аммиака с катионом [Hg2]2+ образует хлорид димеркураммони€ [Hg2NH2]Cl.
2. ’ромат кали€ 2—rO4 дает с катионом [Hg2]2+ красный осадок Hg2CrO4, нерастворимый в гидроксидах и в разбавленной азотной кислоте.
3. ¬осстановление [Hg2]2+ до металлической ртути. ѕри действии на каплю раствора соли ртути(I) 2-3 капл€ми свежеприготовленного раствора хлорида олова SnCl2 образуетс€ белый осадок, который при сто€нии темнеет вследствие восстановлени€ ионов [Hg2]2+ до металлической ртути:
[Hg2]2+ + 2—l- → Hg2Cl2↓
Hg2Cl2+Sn2+ → 2Hg↓ +Sn4+ + 2Cl-
4. Ќа медную пластинку поместите каплю раствора соли ртути (I) и дайте посто€ть. „ерез некоторое врем€ на пластинке образуетс€ серое п€тно Ц металлическа€ ртуть. ќбмойте пластинку водой и протрите тр€почкой или фильтровальной бумагой, оно становитс€ блест€щим:
Hg2(NO3)2+ —u → Cu(NO3)2 + 2Hg↓
”слови€ проведени€ опыта:
1. ћедна€ пластина должна быть предварительно очищена наждачной бумагой.
2. „ерез 2-3 минуты после нанесени€ раствора оксида ртути(I) на пластинку, полученное серое п€тно надо протереть фильтровальной бумагой.
3. —ильные окислители мешают проведению опыта.
4. »оны Hg2+, восстанавливающиес€ этой реакцией, должны быть удалены.(–абота проводитс€ в выт€жном шкафу!)
5. ƒифенилкарбазон с катионом ртути [Hg2]2+ дает соединение фиолетового или синего цвета.
ќпыт: ѕоместите на предметное стекло каплю исследуемого раствора и прибавьте туда каплю 2н раствора азотной кислоты и каплю дифенилкарбазона. ≈сли в растворе имеютс€ катионы [Hg2]2+, то капл€ окрашиваетс€ в синий или фиолетовый цвет. “акую же окраску дают катионы ртути (II) и ионы —rќ42-.
”слови€ проведени€ опыта:
1. атионы Ќg2+ и анионы —rO42 - должны быть удалены.
2. ¬ нейтральных и уксуснокислых растворах катионы Cu2+, Fe2+, Fе3+, Cr3+, Co2+ так же дают окрашенные соединени€.
онтрольна€ задача
“≈ћј: јнализ катионов второй группы
’од анализа: практически нет ни одного реактива, которым можно было бы открыть тот или иной катион второй группы в присутствии других катионов. ѕоэтому необходимо применить систематический ход анализа, т.е. последовательно выделить каждый катион из смеси и затем открыть их.
¬ коническую колбу поместите 20-30 капель исследуемого раствора и, помешива€, добавьте 2н раствор хлороводородной кислоты. „ерез 1-2 минуты осадок отцентрифугируйте и промойте холодной водой, содержащей несколько капель 2н раствора хлороводородной кислоты. ÷ентрифугат и промывные воды не используютс€. ќсадок обработайте 2-3 раза гор€чей водой и центрифугируйте. ѕри этом хлорид свинца переходит в раствор, а хлорид серебра AgCl и хлорид ртути Hg2Cl2 остаетс€ в осадке. ¬ центрифугате открывают катион Pb2+, а в осадке катионы Ag+, [Hg2]2+.
1. ќткрытие катионов Pb2+. 2-3 капл€м центрифугата добавьте такое же количество раствора иодида кали€ Ц образуетс€ осадок иодида свинца PbI2, который при нагревании раствор€етс€, а при охлаждении вновь выпадает в виде золотисто-желтых кристаллов.
2. ќткрытие катиона [Hg2]2+. оставшемус€ осадку в пробирке или на фильтре прилейте 5-7 капель раствора аммиака и перемешайте. ≈сли присутствует катион [Hg2]2+, то осадок чернеет. ’лорид серебра под действием раствора аммиака переходит в раствор в виде комплексной соли, с соль [Hg2NH2]Cl и ртуть остаетс€. ќтделите осадок.
|
|
|
3. ќткрытие катиона јg+. ÷ентрифугат разделите на две части и к одной из них прилейте раствор иодида кали€, а к другой Ц азотной кислоты. ѕри наличии катиона јg+ в первой пробирке выпадет желтый осадок иодида серебра, а во второй Ц белый осадок хлорида серебра. ¬ том и другом случае происходит разрушение комплекса:
[Ag(NH3]2 Cl ↔ [Ag(NH3]2]+ + Cl-
[Ag(NH3]2]+ ↔ 2NH3 + Ag+
2NH3 + Ag+ + 2H+ +Cl- Ѓ AgCl¯ +2NH4+
[Ag(NH3]2 Cl + 2HNO3 Ѓ AgCl¯ + NH4NO3
≈ще более чувствительной реакцией €вл€етс€ реакци€ с (NH4)2S.
Ћабораторна€ работа є3
“≈ћј: “реть€ аналитическа€ группа катионов (анализ катионов группы серной кислоты)
третьей группе относ€тс€ катионы щелочноземельных металлов бари€, стронци€, кальци€. ќни имеют законченные 8 электронные внешние слои. ’имическа€ активность их возрастает от кальци€ к барию. ¬ таком же направлении измен€ютс€ и другие свойства, например растворимость солей, основные свойства гидроксидов и др. »оны этих элементов в водных растворах бесцветны.
»он SO42-c катионами Ba2+, Sr2+, Ca2+, Pb2+ образует осадки, а с катионами других аналитических групп осадка не дает. –азбавленна€ серна€ кислота Ц групповой реагент.
—оли, образованные сильными кислотами, практически не подвергаютс€ гидролизу.
ѕоскольку произведение растворимости CaSO4 равно 9,1×10-6, то осаждение катионов —а2+ разбавленной серной кислотой происходит полностью. „тобы не Употер€тьФ катион —а2+ при анализе, необходимо проводить проверочные реакции на этот катион после осаждени€ катионов третьей группы групповым реагентом или вести осаждение катионов этой группы смесью серной кислоты с этанолом.
ќпыт: ¬озьмите три центрифужные пробирки, прибавьте по 3-5 капель растворов солей: в первую - хлорида бари€, во вторую Ц хлорида стронци€, в третью Ц хлорида кальци€. ƒобавьте в каждую из них по 3 капли раствора серной кислоты, нагрейте на вод€ной бане и наблюдайте за образованием осадков.
— повышением температуры растворимость солей BaSO4, SrSO4, CaSO4 измен€етс€ мало, более полное осаждение происходит при сто€нии в течение 20 минут. ќбратите внимание на скорость выпадени€ осадков при комнатной температуре. ѕроверьте растворимость в сол€ной и азотной кислотах.
ѕосле осаждени€ в пробирку, содержащую сульфат кальци€ добавьте 5 капель ацетона или этилового спирта и проверьте, увеличиваетс€ ли количество осадка. ƒайте объ€снение.
арбонат натри€ и другие растворимые соли угольной кислоты дают с катионами Ba2+, Sr2+, Ca2+ белые осадки, растворимые в сол€ной, азотной и уксусной кислотах. ѕроверьте действие раствора карбоната натри€ Na2CO3 или карбоната аммони€ (NH4)2CO3 на катионы Ba2+, Sr2+, Ca2+ и исследуйте растворимость образовавшихс€ осадков в сол€ной и уксусной кислотах.
„астные реакции катиона ¬а2+
1. ’ромат кали€ 2CrO4 дает с катионом ¬а2+ желтый осадок хромата бари€ ¬а—rO4, нерастворимый в уксусной кислоте, но растворимый в сильных кислотах:
BaCl2 + K2CrO4 Ѓ BaCrO4¯ + 2KCl
Ba2+ + CrO42-Ѓ BaCrO4¯
ќпыт: ¬озьмите в пробирку 3 капли раствора хлорида или нитрата бари€, добавьте 3 капли раствора хромата кали€ и 3 капли раствора уксусной кислоты. Ќагрейте на вод€ной бане. ѕри нагревании выпадет желтый осадок - кристаллический хромат бари€ ¬а—rќ4.
”слови€ проведени€ опыта.
1. –еакци€ проводитс€ в нейтральной или слабокислой среде.
2. ¬ыпадению осадка способствует нагревание.
3. атионы Pb2+ и др., дающие с хромат ионом осадки, мешают проведению реакции.
2. ƒихромат кали€ 2Cr2O7 дает с катионом бари€ также желтый осадок хромата бари€:
2Cr2O7 + BaCl2 + Ќ2ќ Ѓ BaCrO4¯ + 2 —l + 2Ќ—l
Cr2O72- + H2O ↔ 2CrO42- + 2H+
2CrO42- + 2Ba2+ ↔ 2BaCrO4¯
|
|
|
¬ осадок выпадает BaCrO4, а не BaCr2O7, так как в растворе протекает взаимодействие ионов Cr2O72- c водой. онцентраци€ образующихс€ при этом ионов CrO42- вполне достаточна дл€ осаждени€ иона ¬а2+ в виде BaCrO4. роме того, эта реакци€ частично смещаетс€ справа налево ацетатной буферной смесью. ѕри взаимодействии дихромат аниона с водой образуютс€ ионы Ќ+, придающие раствору кислую реакцию. ƒл€ св€зывани€ ионов Ќ+ добавл€ют ацетат натри€:
CH3COONa + H+ Ѓ CH3COOH + Na+
¬ образовавшейс€ уксусной кислоте хромат бари€ не раствор€етс€.
ќпыт. ¬озьмите в пробирку 3 капли раствора хлорида бари€ или нитрата бари€, добавьте 3 капли раствора дихромата кали€ и 4-5 капель раствора ацетата натри€ CH3COONa, нагрейте на вод€ной бане. ѕри этом выпадает кристаллический осадок хромата бари€ BaCrO4.
”слови€ проведени€ опыта:
1. –еакцию следует проводить при избытке ацетата натри€.
2. »оны Sr2+, Ca2+ в присутствии ацетатного буферного раствора не мешает открытию иона ¬а2+.
—ледовательно, дл€ осаждени€ иона ¬а2+ и отделени€ его от катионов стронци€ и кальци€ можно примен€ть как хромат ионы, так и дихромат ионы. ѕроверьте растворимость хромата бари€, стронци€ и кальци€ следующим опытом.
ќпыт: ¬озьмите три пробирки и поместите в каждую по 3 капли растворов хлорида бари€, хлорида стронци€, хлорида кальци€ и добавьте в каждую по капле раствора хромата кали€ и по капле раствора уксусной кислоты. ∆идкости в пробирках перемешайте стекл€нной палочкой и наблюдайте за результатами реакции. ѕроделайте такой же опыт, но вместо уксусной кислоты добавьте 2-3 капли сол€ной кислоты.
3. ќкрашивание пламени. Ћетучие соли бари€ окрашивают бесцветное плам€ в желто-зеленый цвет.
„астные реакции катиона Sr2+
атионы Sr2+ не имеют специфических реакций.
1. √ипсова€ вода (насыщенный водный раствор гипса (CaSO4×2H2O) образует с катионом Sr2+ осадок стронци€.
ќпыт: ¬ пробирку налейте 4 капли раствора нитрата стронци€ Sr(NO3)2, добавьте 5-6 капель гипсовой воды, нагрейте на вод€ной бане и наблюдайте образование осадка.
”слови€ проведени€ опыта:
1. –еакци€ с гипсовой водой может примен€тьс€ при отсутствии катионов ¬а2+, а также катионов, которые дают труднорастворимые осадки с анионом SO42 -.
2. Ќагревание ускор€ет образование осадка.
3. “ак как осадок SrSO4 по€вл€етс€ не сразу, то следует дать посто€ть смеси 10 -15 мин.
2. ќксалат аммони€ (NH4)2C2O4 осаждает катион Sr2+ в виде белого осадка:
Sr(NO3)2 + (NH4)2C2O4 Ѓ SrC2O4¯ + 2NH4NO3
Sr2++ C2O42-Ѓ SrC2O4
3. ќкрашивание пламени. —оли стронци€ окрашивают бесцветное плам€ в карминово-красный цвет.
„астные реакции катиона —а2+
1. ќксалат аммони€ (NH4)2C2O4 (и другие растворимые соли щавелевой кислоты) образуют с катионом —а2+ белый кристаллический осадок:
CaCl2 + (NH4)C2O4 Ѓ CaC2O4¯ + 2NH4Cl
Ca2+ + C2O42-Ѓ CaC2O4
ќпыт: ¬ пробирку налейте 3 капли раствора —aCl2 и добавьте каплю раствора уксусной кислоты, затем прилейте 3 капли оксалата аммони€ и 1-2 капли раствора аммиака. ¬ыпадает белый кристаллический осадок.
”слови€ проведени€ опыта.
1. ¬начале осаждение лучше вести пи рЌ 5-6, а затем при рЌ 7-8.
2. атионы ¬а2+ и Sr2+ мешают проведению реакции.
атионы ¬а2+ и Sr2+ дают так же белые кристаллические осадки с оксалатом аммони€, но произведени€ растворимости этих соединений различны: ѕ–(—а—2ќ4) =2,57×10-9; ѕ–(Sr—2ќ4) =5,6×10-8; ѕ–(¬а—2ќ4)= 1,6×10-7.—ледовательно, осадок оксалата кальци€ можно считать практически нерастворимым в воде. –астворимость оксалатов в уксусной кислоте различна. ќксалат кальци€ нерастворим, оксалат бари€ раствор€етс€ в уксусной кислоте несколько в большей степени, чем оксалат стронци€.
ќксалаты бари€, стронци€, кальци€ растворимы в сильных минеральных кислотах (сол€ной и азотной).
ќпыт: ¬озьмите три пробирки, в каждую налейте по 3 капли растворов хлорида бари€, хлорида стронци€, хлорида кальци€ и прилейте в каждую по 3-4 капли раствора оксалата аммони€. Ќаблюдайте образование осадка. ѕосле этого добавьте в каждую пробирку по 6 капель раствора уксусной кислоты и посмотрите, где будет происходить частичное растворение осадка. «атем повторите опыт, но вместо уксусной кислоты добавьте 6 капель раствора сол€ной кислоты. ќтметьте, что происходит с осадками.
2. ћикрокристаллоскопическа€ реакци€. ќбразование осадка сульфата кальци€ —аSќ4 × 2Ќ2ќ при взаимодействии катиона —а2+ с серной кислотой можно легко заметить, если проводить реакцию на предметном стекле, наблюда€ выпавшие кристаллы под микроскопом.
ќпыт: ќдну каплю раствора соли кальци€ поместите на предметное стекло, добавьте каплю 2Ќ раствора серной кислоты и осторожно нагрейте на маленьком пламени газовой горелки до по€влени€ белой каймы. ѕо кра€м капли образуютс€ игольчатые кристаллы в виде пучков или звездочек.
|
|
|
ѕри наличии в растворе катионов ¬а2+ и Sr2+ поступают следующим образом: в пробирку внос€т 3-4 капли испытуемого раствора, добавл€ют 4 капли 2Ќ раствора серной кислоты, нагревают 5-6 минут на вод€ной бане, при этом ионы ¬а2+ и Sr2+ переход€т в осадок, а ионы —а2+ остаютс€ в растворе. ќсадок отдел€ют фильтрованием. аплю фильтрата помещают на предметное стекло и упаривают до по€влени€ белой каймы, а затем рассматривают полученные кристаллы под микроскопом.
3. ќкрашивание пламени. Ћетучие соли кальци€ окрашивают бесцветное плам€ горелки в кирпично-красный цвет.
онтрольна€ задача
“≈ћј: јнализ катионов третьей группы
ѕредварительные испытани€.
анализируемому веществу прибавл€ем по капл€м гипсовую воду и наблюдаем за выпадением осадка.
| Ёффект | катион | ||
| ¬а2+ | Sr2+ | Ca2+ | |
| ќсадок не образуетс€ | отсутствует | отсутствует | присутствует (дл€ проверки прово-д€т реакцию с родизонатом Na |
| ќсадок по€вл€етс€ постепенно | ___ | присутствует | может присутствовать |
| ќсадок образуетс€ мгновенно | присутствует | может присутствовать | ћожет присутствовать |
’од анализа:
¬виду того, что бихромат кали€ 2Cr2ќ7 дает в уксуснокислой среде осадок только с катионом ¬а2+, то обычно катионы ¬а2+ отдел€ют от катионов Sr2+ и —а2+, добавл€€ к испытуемому раствору ацетат натри€ и раствор дихромата кали€. ѕри этом катионы ¬а2+ переход€т в осадок в виде ¬а—rќ4, а катионы Sr2+ и —а2+ и избыток хромат-ионов —rќ42- остаетс€ в растворе. ќсадок отфильтровывают и провер€ют полноту отделени€ бари€.
части фильтрата добавл€ют гипсовую воду и нагревают на вод€ной бане 7-10 минут; если при сто€нии выпадает осадок, то в растворе наход€тс€ катионы стронци€.
≈сли в растворе есть катионы стронци€, то к центрифугату (фильтрату), который не содержит гипсовой воды, добавл€ют раствор карбоната натри€ и отдел€ют осадок карбонатов SrCO3 и —а—ќ3, промывают его два раза дистиллированной водой, раствор€ют в уксусной кислоте. раствору добавл€ют сульфат аммони€, при этом катионы стронци€ образуют осадок сульфата стронци€ SrSO4, а катионы —а2+ (в значительной мере) остаютс€ в растворе в виде комплексной соли (NH4)2[Ca(SO4)2].
ќбнаружение и отделение катионов ¬а2+. ¬ коническую пробирку берут 3 капли испытуемого раствора, прибавл€ют 2-4 капли ацетата натри€ и 3 капли раствора дихромата кали€. ≈сли осадок образуетс€, то это указывает на присутствие катионов ¬а2+. ƒл€ удалени€ катионов бари€ берут в коническую пробирку 5-6 капель анализируемого раствора, 5-6 капель раствора уксусной кислоты и 5-6 капель дихромата кали€, перемешивают стекл€нной палочкой, дают сто€ть 2-3 минуты и отдел€ют осадок (фильтрованием).
ќбнаружение и отделение катионов Sr2+. ‘ильтрат после проверки на полноту осаждени€ катионов бари€ в количестве 2-3 капель помещают в пробирку, при помощи пипетки добавл€ют 2-3 капли гипсовой воды, нагревают на вод€ной бане до 70∞— и дают сто€ть 15-20 минут. ќбразование осадка говорит о присутствии в испытуемом растворе катионов Sr2+. ѕоследний провер€ют на плам€. арминово-красное окрашивание указывает на присутствие катионов стронци€.






