ѕри диссоциации слабого бинарного электролита в растворе устанавливаетс€ равновесие.
CH3COOH  †CH3COO- + H+
†CH3COO- + H+
ѕри этом, если начальна€ концентраци€ электролита равна —, а степень диссоциации a, то

[CH3COO-] = [H+] = aC
[CH3COO-] = C - aC

дл€ a<<1†††††††† K=ca2††††† †и††† 
ѕриведенное уравнение выражает закон разбавлени€ ќствальда, согласно которому степень диссоциации слабого электролита растет с разбавлением раствора.
ƒобавление в раствор слабых электролитов одноименных ионов вызывает смещение равновеси€ реакции диссоциации в сторону ее уменьшени€ (эффект одноименного иона).
Ёлектролитическа€ диссоциаци€ воды
ѕроцесс ионизации воды протекает по уравнению:
Ќ2ќ  †Ќ+ + ќЌ-- + 55,90 кƒж/моль.
†Ќ+ + ќЌ-- + 55,90 кƒж/моль.
онстанта равновеси€ процесса диссоциации воды можно записать в виде:

онцентраци€ молекул воды Ц посто€нна€ величина, которую можно рассчитать по уравнению:
†[H2O]= n(H2O)/1л = 1000  0,9971/18,015 = 55,5 моль/л,
0,9971/18,015 = 55,5 моль/л,
где 0,9971г/мл -плотность воды, 18,015 г/моль -мол€рна€ масса воды.
ќбъедин€€ две посто€нные величины в одной части уравнени€, получим:
K[H2O] = 1,8×10-16 × 55,5 = 10-14 = [H+] × [OH-] = KH2O Ц ионно円†††††††††††††††††††††††††††††††††††††††††††††††††††† произведение воды.
»онное произведение воды увеличиваетс€ с увеличением температуры:
| t,∞C | 10 | 22 | 30 | 100 |
| KH2O | 0,4×10-14 | 1,0×10-14 | 1,9×10-14 | 74×10-14 |
¬ нейтральном растворе концентрации ионов водорода и гидроксид-ионов равны: [H+] = [OH-] =  = 10-7 моль/л.
= 10-7 моль/л.
¬ кислом растворе [Ќ+] > [OH-]; [H+] >10-7 моль/л.
†¬ щелочном растворе [H+] < [OH-]; [H+]< 10-7 моль/л.
«на€ концентрацию одного из ионов, например [Ќ+] и ионное произведение воды, можно рассчитать концентрацию ионов [OH-] и, наоборот.
ѕользоватьс€ в расчетах такими малыми величинами концентраций ионов(10-9, 10-13 моль/л и т.д.) неудобно, поэтому используют их отрицательные дес€тичные логарифмы. ќтрицательный логарифм концентрации ионов водорода (или отрицательный логарифм активности ионов водорода) называют водородным показателем,рЌ:
†рЌ = Цlg[H+]††
«на€, что [H+] × [OH-]= 10-14, получим: рЌ + рќЌ = 14
¬ нейтральном растворе при 22о— рЌ = рќЌ = 7.
¬ кислом растворе рЌ < 7.
¬ щелочном растворе рЌ > 7.
ислотно-основные индикаторы Ц это вещества, мен€ющее окраску в определенной области значени€ ph раствора. »ндикаторами могут быть слабые органические кислоты или основани€, молекулы и ионы которых имеют разную окраску.
ќбласть перехода окраски некоторых индикаторов
|
»ндикатор | † ÷вет | ќбласть перехода окраски, рЌ | |
| †кислотна€ форма | †щелочна€ форма | ||
| ћетилоранж | красный | желтый | 3,2 Ц 4,5 |
| ‘енолфталеин | бесцветный. | красный | 8,2 Ц 10,0 |
| Ћакмус | красный | синий | 6,0 Ц9,0 |
Ѕуферные растворы
Ѕуферные растворы используют дл€ поддержани€ посто€нной величины рЌ в исследуемом растворе при добавлении к нему небольших количеств сильной кислоты, сильного основани€ или при разбавлени€ раствора.
|
|
|
¬ качестве буферных растворов обычно используют смеси растворов слабых кислот или слабых оснований и их солей или смеси солей многоосновных кислот различной степени замещени€. ¬ таблице приведены примеры наиболее часто используемых буферных растворов и величины рЌ, которые они поддерживают:
| —остав буферного раствора | Ќазвание буфера | рЌ |
| —месь —Ќ3—ќќЌ и —Ќ3—ќќNа | јцетатный буфер | 4,7 |
| —месь NаЌ2–ќ4 и Nа2Ќ–ќ4 | ‘осфатный буфер | 6,5 |
| —месь NЌ4ќЌ и NЌ4—1 | јммиачный буфер | 9,25 |
Ѕуферна€ система может св€зывать как ионы Ќ+, так и ќЌ- приливаемых сильных кислот и оснований в слабые электролиты, незначительно измен€€ величину рЌ раствора.
ѕример: јцетатный буферный раствор содержит смесь CH3COOH и CH3COONa. ƒиссоциаци€ слабого электролита Ц уксусной кислоты Ц отражаетс€ уравнением реакции:††††††††††††††††††††††† CH3COOH  †CH3COO
†CH3COO  †+ H+ и описываетс€ константой равновеси€:
†+ H+ и описываетс€ константой равновеси€:
Ka=  †= 1.8 10
†= 1.8 10 
ѕри добавлении ацетата натри€ концентраци€ ионов CH3COO  †возрастает и определ€етс€ концентрацией соли: [CH3COO
†возрастает и определ€етс€ концентрацией соли: [CH3COO  ]
]  Cс. ƒиссоциаци€ слабого электролита уменьшаетс€ за счет введени€ одноименного иона, поэтому [CH3COOH]
Cс. ƒиссоциаци€ слабого электролита уменьшаетс€ за счет введени€ одноименного иона, поэтому [CH3COOH]  Cк, где Cк Ц концентраци€ кислоты.
Cк, где Cк Ц концентраци€ кислоты.
Ka= 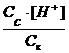 ;††
;††
[H+] = Ka  ;††
;††
pH = Цlg[H+] = pKa Ц lg ( ),†
),†
где pKa = - lg Ka.
“аким образом, рЌ буферных растворов не зависит от концентраций компонентов, а определ€етс€ их отношением.
ѕри добавлении небольших количеств сильных кислот и оснований компоненты буферного раствора реагируют с ними, перевод€ их в слабые электролиты:
CH3COOH + NaOH = CH3COONa + H2O (концентраци€ соли увеличиваетс€ на концентрацию добавленной щелочи, а концентраци€ кислоты уменьшаетс€ на ту же величину):
pH = Цlg[H+] = pKa - lg ( );
);
CH3COONa + HCl = CH3COOH + NaCl,
pH = Цlg[H+] = pKa - lg ( ).
).
“ак как отношение концентраций измен€етс€ меньше, чем их сумма или разность, общее значение рЌ измен€етс€ незначительно.
оличество сильной кислоты или сильного основани€, которые нужно добавить к буферному раствору дл€ изменени€ рЌ одного литра его раствора на единицу, называют буферной емкостью (B). ќна может быть вычислена относительно кислоты (B а) или основани€ (Bb).
Ba = 
Bb = 
где Ba и Bb Ц буферные емкости по кислоте и основанию соответственно; Ca и Cb Ц концентрации добавленных кислоты и основани€; pH 1 и pH 2 Ц исходные и конечные значени€ рЌ раствора; † Va and Vb Ц объемы добавленных сильных кислоты и основани€.
ѕримеры решени€ задач
ѕример 26. –ассчитайте pH 0.01 M раствора NaOH.
–ешение. “ак как NaOH €вл€етс€ сильным электролитом, то он полностью диссоциирует в растворах:
NaOH Ѓ Na+ + OH-
[OH-] = C(NaOH) = 10-2 моль/л.
[H+] = 10-14/[OH-] = 10-12 моль/л.
pH = Цlg [H+] = 12.
ѕример 27. –ассчитайте pH 0.01 M раствора of CH3COOH.
–ешение. ”ксусна€ кислота Ц слабый электролит, диссоциирующий обратимо: CH3COOH  ††CH3COO- + H+
††CH3COO- + H+
[H+] = a×C(CH3COOH)
—огласно закону разбавлени€ ќствальда,  , поэтому
, поэтому 
онстанта диссоциации уксусной кислотыЦ таблична€ величина, котора€ равна K(CH3COOH) = 1.75×10-5, поэтому
 †моль/л
†моль/л
pH = Цlg [H+] = Цlg (4.18×10-4) = 4 Ц lg(4.18) = 3.38.
ѕример 28. –ассчитайте pH буферного раствора, содержащего 0.01 моль/л CH3COOH и 0.1 моль/л CH3COONa.
|
|
|
–ешение. †††††††CH3COOH  ††CH3COO- + H+
††CH3COO- + H+
(слабый электролит)
CH3COONa Ѓ CH3COO- + Na+
(сильный электролит)


pH = Цlg [H+] = Цlg (1.75×10-6) = 6 Ц lg(1.75) = 5.76.






