Ќаправление ионных реакций
–еакции в растворах электролитов протекают между ионами и идут в сторону образовани€ газообразных или малорастворимых веществ (см. прил., табл. 6), или слабых электролитов.
Ќапример, при добавлении щелочи в раствор хлорида меди образуетс€ осадок Cu(OH)2:
CuCl2 + 2NaOH = Cu(OH)2¯+ 2NaCl,
Cu2+ + 2Cl-+ 2Na++ 2OH-= Cu(OH)2¯ + 2Na++ 2Cl-.
(полное ионно-молекул€рное уравнение)
–авновесие сдвигаетс€ вправо из-за образовани€ осадка Cu(OH)2. —окращенное ионное уравнение отражает сущность ионной реакции:
Cu 2+ + 2OH- = Cu(OH)2¯.
ѕример реакции, котора€ протекает в сторону образовани€ одновременно газообразного продукта CO2 и мало диссоциирующего вещества Ќ2ќ:
K2CO3 + 2HCl = 2KCl + H2O + CO2≠;
2K++ CO32-+ 2H++ 2Cl- = 2K++ 2Cl-+ H2O + CO2≠;
CO32-+ 2H+ = H2O + CO2≠.
ѕример ионной реакции, протекающей в сторону образовани€ мало диссоциирующего электролита, например H2O:
2NaOH + H2SO4 = Na2SO4 + 2H2O;
2Na++ 2OH-+ 2H++ SO42-= 2Na+ + SO42-+ 2H2O;
H+ + OH- = H2O.
¬ ионных или ионно-молекул€рных уравнени€х реакций с участием электролитов сильные растворимые электролиты, поскольку они полностью диссоциированы, записывают в виде ионов, а слабые электролиты, малорастворимые и газообразные вещества, записывают в виде молекул.
—уммы электрических зар€дов в левой и правой част€х ионно-молекул€рных уравнений должны быть равны.
»онное произведение воды
¬ода €вл€етс€ очень слабым электролитом, диссоциирующим по уравнению Ќ2ќ ЂЌ++ ќЌ-.
онстанта диссоциации воды при 22∞— равна:
K д =  = 1,8 × 10-16.
= 1,8 × 10-16.
ƒол€ молекул воды, диссоциированных на ионы, настолько мала, что концентрацию недиссоциированных молекул можно считать посто€нной и равной числу молей воды в литре, т.е.
[H2O] =  = 55,56 моль / л.
= 55,56 моль / л.
ћол€рна€ масса воды равна 18 г/моль.
ѕодставив это значение в выражение константы диссоциации, получим
[H+] × [OH-] = 1,8 × 10-16×55,56 = 10-14.
Ёта посто€нна€ величина называетс€ ионным произведением воды ( Ќ2ќ); она не зависит от присутстви€ в воде других ионов (но зависит от температуры). ¬с€кий водный раствор, как и чиста€ вода, содержит ионы Ќ+и ќЌ-и ни в каком случае [H+] или [OH-] не могут быть равны нулю, так как при этом их произведение должно обратитьс€ в нуль.
ѕри растворении в воде какой-либо кислоты концентраци€ катионов водорода [H+] увеличиваетс€, а концентраци€ [OH-] уменьшаетс€, и наоборот. ¬еличина ионного произведени€ при этом посто€нна€ [H+] × [OH-] = 10-14.
ѕри диссоциации чистой воды
[H+] = [OH-] = 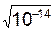 =1×10-7моль/л.
=1×10-7моль/л.
¬ кислой среде [H+]>[OH-], [H+]>1∙10-7 моль/л и соответственно [OH-]<1∙10-7 моль/л. Ќапример, при [HCl]=0,1 моль/л рЌ=1.
¬ щелочной среде [H+]<[OH-], [H+]<1∙10-7 моль/л и соответственно [OH-]>1∙10-7 моль/л. Ќапример, при [KOH]=0,1 моль/л рЌ=13.






