— Ћјƒ” –ќ«„»Ќ≤¬
«авданн€ 1. ¬ 1 кг води розчинили 200 г натр≥й г≥дроксиду.
ќбчислити мол€льну концентрац≥ю цього розчину, мольну ≥ масову частку розчиненоњ речовини.
–озвТ€зок
ћол€льна концентрац≥€ - це в≥дношенн€ к≥лькост≥ речовини до маси розчинника в кг.
 , де:
, де:  , тому
, тому  .
.
M(NaOH) = 23 + 16 + 1 = 40 (г/моль).
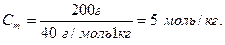
ћольна частка розчиненоњ речовини дор≥внюЇ в≥дношенню к≥лькост≥ розчиненоњ речовини до суми к≥лькост≥ речовини вс≥х компонент≥в розчину.
 , де:
, де: 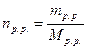 ,
,  .
.
M(NaOH) = 23 + 16 + 1 = 40 (г/моль)
ћ(Ќ2ќ) = 2 . 1+16 = 18 (г/моль)
«в≥дси 

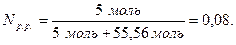
ћасова частка розчиненоњ речовини Ц це в≥дношенн€ маси розчиненоњ речовини до маси розчину, виражене у в≥дсотках.

mр-ну = mp.p. + mр-ка; mр-ка = р . V.
mр-ка = 1  . 1000
. 1000 
 = 1000
= 1000 
mр-ну = 40  +1000
+1000  = 1400
= 1400 
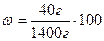 % = 2,86%
% = 2,86%
«авданн€ 2. ќбчислити масу натр≥й карбонату Na2CO3, що м≥ститьс€ в 500 мл розчину, мол€рна концентрац≥€ екв≥валента €кого 0,3 моль/л.
–озвТ€зок
ћол€рна концентрац≥€ екв≥валента Ц це в≥дношенн€ к≥лькост≥ речовини екв≥валента до обТЇму розчину.
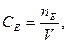 де:
де:  ;
; 
≈кв≥валентне число z дл€ сол≥ дор≥внюЇ добутку валентност≥ елемента на число його атом≥в в сол≥.
ќтже, 
«в≥дси ћ≈ (Na2CO3) = ½ . (23.2+12+16.3)=53 (г/моль).
« формули  визначимо масу розчиненоњ речовини:
визначимо масу розчиненоњ речовини:
m = C≈ .ћ≈ .V; m = 0,3 моль/л Ј 53 г/моль Ј 0,5 л = 7,95 г.
“≈ћј 5. –≈ј ÷≤ѓ ќЅћ≤Ќ” ¬ –ќ«„»Ќј’
≈Ћ≈ “–ќЋ≤“≤¬
«авданн€ 1. Ќаписати молекул€рне, йонно-молекул€рне ≥ скорочене йонне р≥вн€нн€ реакц≥њ м≥ж натр≥й карбонатом ≥ сульфатною кислотою.
–озвТ€зок
«апишемо молекул€рне р≥вн€нн€:
 H2O
H2O
 Na2CO3+ H2SO4 = Na2SO4+H2CO3
Na2CO3+ H2SO4 = Na2SO4+H2CO3
CO2
Na2CO3, H2SO4 ≥ Na2SO4 - сильн≥ електрол≥ти, а H2CO3 - слабкий, електрол≥т, що розкладаЇтьс€ на воду ≥ вуглекислий газ.
¬с≥ сильн≥ електрол≥ти запишемо в йонному вид≥, продукти розкладу слабкого електрол≥ту Ц в молекул€рн≥й форм≥^
 H2O
H2O
 2 Na + +CO32- + 2H+ + SO 42- = 2Na+ + H2CO3
2 Na + +CO32- + 2H+ + SO 42- = 2Na+ + H2CO3
CO2↑ 
¬иключимо под≥бн≥ йони, що не беруть участ≥ в реакц≥њ, ≥ одержимо скорочене йонне р≥вн€нн€:
 Ќ2ќ
Ќ2ќ
 2Ќ+ + —ќ32- = Ќ2—ќ3
2Ќ+ + —ќ32- = Ќ2—ќ3
—ќ2↑
–еагують сильн≥ електрол≥ти, а в результат≥ реакц≥њ утворюЇтьс€ слабкий електрол≥т.
ќтже, ц€ реакц≥€ йде до к≥нц€.
“≈ћј 6. ƒ»—ќ÷≤ј÷≤я ¬ќƒ». ¬ќƒЌ≈¬»… ѕќ ј«Ќ»
«авданн€ 1. ¬изначити концентрац≥ю йон≥в √≥дрогену в розчин≥, рЌ €кого дор≥внюЇ 6,3.
–озвТ€зок
« умови завданн€ маЇмо: рЌ = -Ig[H+] = 6,3 або lg[H+] = -6,3; [H+] = 10-6,3; [H+] = 5. 10-7 моль/л.
«авданн€ 2. онцентрац≥€ йон≥в Ќ+ в грунтовому розчин≥ сильно кислих п≥дзолистих грунт≥в дор≥внюЇ 10-3 - 10-4 моль/л.
¬изначити концентрац≥ю йон≥в ќЌ -, рЌ ≥ рќЌ розчину.
–озвТ€зок
« формули йонного добутку води 
визначаЇмо концентрац≥ю г≥дроксид-йон≥в:
 ;
;
 моль/л
моль/л
 моль/л
моль/л
«а концентрац≥Їю йон≥в Ќ+ визначаЇмо рЌ:
pH= -Ig [H+]:
pH1 = -Ig10 -3 = 3; pH2 = -Ig10 -4 = 4.
«а концентрац≥Їю йон≥в ќЌ - визначаЇмо pOH:
pOH = -Ig[OH];pOH1 = -Ig10 -11 = 11; pOH2 = -Ig10 -10 = 10.
“≈ћј 7. √≤ƒ–ќЋ≤« —ќЋ≈…
«авданн€ 1. исл≥ болотн≥ грунти мають високий вм≥ст алюм≥н≥й хлориду, €кий знижуЇ рЌ ≥ пог≥ршуЇ структуру грунт≥в. „им це по€снюЇтьс€?
|
|
|
–озвТ€зок
јлюм≥н≥й хлорид ј≤—≤3 Ц це с≥ль, утворена слабкою основою ≥ сильною кислотою, тому вона г≥дрол≥зуЇтьс€. √≥дрол≥з в≥дбуваЇтьс€ за кат≥оном, ≥ в розчин≥ накопичуютьс€ йони √≥дрогену, €к≥ спричин€ють зниженн€ водневого показника рЌ:
ј≤—≤3 = ј≤+3 +3—≤-
ј≤3+ + ЌќЌ L ј≤ќЌ2+ +Ќ+, рЌ < 7, в молекул€рн≥й форм≥.
ј≤—≤3 + ЌќЌ L ј≤ќЌ—≤2 + Ќ—≤.
«авданн€ 2. ¬ грунтовому розчин≥ солончак≥в м≥ститьс€ натр≥й карбонат Na2CO3 (сода). „ому ц≥ грунти мають лужну реакц≥ю?
–озвТ€зок
—ода Na2CO3 Ц це с≥ль, утворена сильною основою ≥ слабкою кислотою, тому вона г≥дрол≥зуЇтьс€. √≥дрол≥з в≥дбуваЇтьс€ за ан≥оном, ≥ в розчин≥ накопичуютьс€ йони ќЌ -, €к≥ спричин€ють лужну реакц≥ю.
Na2CO3 = 2Na+ + CO32-
—ќ32- + ЌќЌ L Ќ—ќ3- + ќЌ- або в молекул€рн≥й форм≥
Na2CO3 + H2O L NaHCO3 + NaOH; рЌ > 7 (лужне cередовище).
“≈ћј 8. ќ »—Ќќ-¬≤ƒЌќ¬Ќ≤ –≈ј ÷≤ѓ
«авданн€ 1. Ћужн≥сть ірунтового розчину може виникнути внасл≥док життЇд≥€льност≥ сульфатредукуючих бактер≥й, €к≥ в≥дновлюють натр≥й сульфат в анаеробних умовах за схемою:
Na2SO4 + C → CO2 + Na2S;
Na2S + CO2 + H2O → Na2CO3 + H2S.
ƒо €ких тип≥в належать ц≥ реакц≥њ?
ƒл€ окисно-в≥дновноњ реакц≥њ скласти електронн≥ р≥вн€нн€, розставити коеф≥ц≥Їнти ≥ визначити мол€рн≥ маси екв≥валент≥в окисника та в≥дновника.
–озвТ€зок
¬изначаЇмо ступен≥ окисненн€ елемент≥в в обох реакц≥€х:


¬ першому випадку зм≥нюютьс€ ступен≥ окисненн€ елемент≥в, в другому - ступен≥ окисленн€ залишаютьс€ незм≥нними.
ќтже, перша реакц≥€ - окисно-в≥дновна, друга - не належить до окисно-в≥дновних.
¬ окисно-в≥дновн≥й реакц≥њ зм≥нюють ступ≥нь окисненн€ —ульфур ≥ арбон.
¬изначаЇмо окисник ≥ в≥дновник.
ќкисник Ц це речовина, атоми або йони €коњ приЇднують електрони, а в≥дновник Ц це речовина, атоми або йони €коњ в≥ддають електрони. ќтже, окисник Ц Na2SO4, в≥дновник Ц —.
—кладаЇмо електронн≥ р≥вн€нн€ ≥ визначаЇмо процеси в≥дновленн€ ≥ окисненн€.
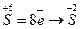 - в≥дновленн€-приЇднанн€ електрон≥в;
- в≥дновленн€-приЇднанн€ електрон≥в;
 - окисненн€-втрата електрон≥в.
- окисненн€-втрата електрон≥в.
¬изначаЇмо найменше сп≥льне кратне дл€ к≥лькост≥ в≥дданих ≥ приЇднаних електрон≥в та одержуЇмо коеф≥ц≥Їнти дл€ окисника ≥ в≥дновника.
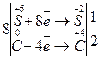
«а коеф≥ц≥Їнтами л≥воњ частини р≥вн€нн€ ставимо коеф≥ц≥Їнти в праву частину:
Na2SO4 + 2C = 2CO2 + Na2S.
«авданн€ 2. ¬изначити екв≥валенти ≥ мол€рн≥ маси екв≥валент≥в окисника ≥ в≥дновника в так≥й окисно-в≥дновн≥й реакц≥њ:
H2S + H2SO3 = S + H2O.
–озвТ€зок

в≥дновник окисник

…он S2- в≥ддаЇ 2 електрони, тому f≈ ¬ дл€ H2S дор≥внюЇ 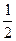 , а екв≥валент - -
, а екв≥валент - - 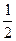 H2S. …он SO32- приймаЇ 4 електрони, тому f≈ ¬ дл€
H2S. …он SO32- приймаЇ 4 електрони, тому f≈ ¬ дл€
H2SO3 дор≥внюЇ  , а екв≥валент -
, а екв≥валент -  H2SO3.
H2SO3.
ћол€рна маса екв≥валента в≥дновника H2S:

ћол€рна маса екв≥валента окисника H2SO3:







