–астворы с точно известной мол€рной концентрацией эквивалента растворенного вещества называют стандартными растворами. ѕриготовить стандартный раствор можно одним из следующих способов:
а) по точной навеске вещества. Ќавеска Ц точно взвешенна€ масса вещества.Ќавеску вещества берут следующим образом: на аналитических весах взвешивают пустой бюкс, помещают в него вещество и взвешивают бюкс с веществом. «атем вещество перенос€т в мерную колбу и еще раз взвешивают пустой бюкс с остатками вещества. Ќаход€т точную массу вещества Ц это и есть навеска. ¬место бюкса в некоторых случа€х можно использовать часовое стекло или кальку.
ƒл€ приготовлени€ стандартного раствора определенную навеску вещества раствор€ют сначала в небольшом объеме воды (или другого растворител€), затем объем раствора в колбе довод€т до метки водой или другим растворителем. –ассчитывают мол€рную концентрацию эквивалента вещества в растворе.
ѕриготовить стандартный раствор по точной навеске можно лишь дл€ тех веществ, которые удовлетвор€ют следующим требовани€м:
1) вещество €вл€етс€ химически чистым;
2) состав вещества строго соответствует химической формуле;
3) вещество не взаимодействует с кислородом, углекислым газом воздуха, не поглощает влагу.
б) из фиксанала. ‘иксанал представл€ет собой запа€нную стекл€нную ампулу, содержащую определенное количество вещества в сухом виде или в растворе, при приготовлении из которого 1 дм3 водного раствора мол€рна€ концентраци€ эквивалента вещества в растворе будет точно равна указанной на ампуле.
»зготовл€ют фиксаналы в специальных лаборатори€х. ƒл€ приготовлени€ стандартного раствора ампулу разбивают определенным образом, содержимое ампулы количественно перенос€т в мерную колбу и разбавл€ют водой до метки. — учетом указанной на ампуле концентрации вещества и вместимости мерной колбы рассчитывают концентрацию полученного раствора.
ѕриготовление стандартного раствора из фиксанала используют в тех случа€х, когда не представл€етс€ возможным вз€ть точную навеску вещества, например, в полевой лаборатории, в геологической экспедиции, а также при выполнении экспресс-анализов.
в) по первичному стандарту. –абочие растворы реагентов готов€т сначала приблизительно заданной концентрации, а затем их титруют стандартными растворами соответствующих реагентов и по результатам титровани€ определ€ют точную концентрацию рабочих растворов. “о вещество, которое используетс€ дл€ пр€мого или косвенного определени€ концентрации рабочего раствора реагента (стандартизации раствора) называетс€ первичным стандартом. ¬ качестве первичных стандартов рассматриваютс€ только те вещества, которые удовлетвор€ют р€ду важнейших требований:
1. »меют самую высокую степень чистоты, и, более того, должны существовать доступные и надежные методы, подтверждающие их чистоту.
2. явл€ютс€ химически устойчивыми к воздействию компонентов атмосферы.
3. Ќе содержат гидратную воду. √идроскопичное или склонное к выветриванию вещество трудно высушить и взвесить.
4. явл€ютс€ легкодоступными.
|
|
|
5. »меют достаточно высокую мол€рную массу эквивалента. „ем выше эта масса, тем больше навеска вещества, требующегос€ дл€ стандартизации или приготовлени€ раствора заданной концентрации по точной навеске; ошибка взвешивани€ при этом уменьшаетс€.
„исло веществ, которые могут быть использованы в качестве первичных стандартов, ограничено. „аще всего дл€ стандартизации используют так называемые вторичные стандарты Ц вещества менее чистые, но имеющие посто€нный химический состав.
ќЅ”„јёў»≈ «јƒј„»
–асчеты при приготовлении раствора заданного состава, его разбавлении, концентрировании, смешивании растворов одного и того же вещества объедин€ет одна обща€ иде€ Ц во всех случа€х записывают уравнени€ материального баланса, св€зывающие характеристики конечного раствора и его исходных составл€ющих. ¬ результате получают систему из двух алгебраических уравнений, реша€ которую, определ€ют искомые величины, необходимые дл€ приготовлени€ раствора с заданными характеристиками.
¬ расчетах с использованием массовой доли w (ј) основу составл€ют два закона сохранени€ масс Ц относительно массы раствора (смеси) и относительно массы растворенного вещества, составленные на основе характеристик составных частей I и II, а также самого раствора (смеси):
1) баланс по массе раствора (смеси):
mр-ра(I) + mр-ра(II) = m(р-ра) (или m(смеси))
2) баланс по массе растворенного вещества ј:
mA(I) + mA(II) = mA(р-р) (или mA(смеси))
ƒалее величины, представленные в этих уравнени€х, выражают согласно условию задачи через объемы, плотности и массовые доли растворенного вещества соответствующих растворов I и II (соответствующих компонентов I и II) раствора.
—ледует отметить, что закон сохранени€ объема при смешивании растворов в общем виде не выполн€етс€, то есть объем смеси не всегда равен сумме объемов исходных растворов.
¬ расчетах с использованием мол€рной концентрации с(ј) основу составл€ют закон сохранени€ количества растворенного вещества и приближенно выполн€ющийс€ закон сохранени€ объема раствора (смеси) в случае разбавлени€ и смешени€ растворов. ѕоследний выполн€етс€ тем точнее, чем более разбавлены смешиваемые растворы:
1) баланс по объему смеси при разбавлении и смешивании:
Vр-ра(I) + Vр-ра(II) = Vсмеси
2) баланс по количеству растворенного вещества:
nј(I) + nј(II) = nј(смеси)
или сј(I)Vр-ра(I) + сј(II)Vр-ра(II) = сј(смесь)V(смесь)
ѕример 1.
акие количества составных частей I и II необходимо вз€ть дл€ приготовлени€ 220 см3 раствора хлорида кальци€ с w (CaCl2)= 8 %, плотность раствора r = 1,066 г/мл. –ассмотреть следующие способы приготовлени€ раствора:
а) m(CaCl2)(тв) и V(H2O) Ц растворение CaCl2(тв) в воде;
б) m(CaCl2Ј6H2O) и V(H2O) Ц растворение кристаллогидрата CaCl2Ј6H2O в воде;
в) m (CaCl2)(тв) + Vр-ра с w (—aCl2) =2 % и r =1,015 г/см3 Ц концентрирование раствора
г) Vр-ра с w (—aCl2) =25 % и r =1,228 г/см3 и V(Ќ2ќ) Ц разбавление концентри≠рованного раствора;
д) Vр-ра с w (—aCl2) =25 % и r =1,228 г/см3 и Vр-ра с w (—aCl2)= 2 % и r =1,015 г/см3 Ц смешивание двух растворов;
е) Vр-ра с w (—aCl2)= 2 % и r =1,015 г/см3  концентрирование раствора путем упаривани€ растворител€.
концентрирование раствора путем упаривани€ растворител€.
–ешение.
а) —оставим два балансовых уравнени€
1)по массе раствора:
m (CaCl2) + m (Ќ2ќ) = m (р-ра) = VЈr
2)по массе растворенного вещества:
M (CaCl2) = VЈrЈw (—aCl2)
ѕодставл€ем данные из услови€ задачи и получаем систему двух уравнений:
|
|
|
m (CaCl2) + m (Ќ2ќ) = 220 см3 Ј1,066 г/см3 =234,52 г
m (CaCl2) = 220 см3 Ј1,066 г/см3 Ј0,08=18,76 г
ќтсюда m (H2O)= 234,52 г Ц 18,76 г = 215,76 г
ќтвет: дл€ приготовлени€ раствора необходимо 18,76 г CaCl2(тв) и 215,76 см3 Ќ2ќ
(при условии, что r =1 г/см3).
б) –ассужда€ подобным образом:
1) ”равнение материального баланса по массе раствора:
m (CaCl2Ј6Ќ2ќ) + m (Ќ2ќ) = m (р-ра) = VЈr
2) ”равнение материального баланса по массе вещества CaCl2 в растворе:
m (CaCl2/ CaCl2Ј 6H2O) = VЈrЈw (—aCl2),
где m (CaCl2/CaCl2Ј 6H2O) = 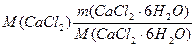 из соображени€, что n(CaCl2) = n(CaCl2Ј6H2O)
из соображени€, что n(CaCl2) = n(CaCl2Ј6H2O)
—истема уравнений принимает вид: m(CaCl2Ј 6H2O) + m(Ќ2ќ) = m(р-ра) = VЈr
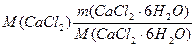 = VЈrЈw(—aCl2),
= VЈrЈw(—aCl2),
подставл€ем данные из услови€ задачи:
m (CaCl2Ј 6H2O) + m(Ќ2ќ) = 220 см3 Ј1,066 г/см3 =234,52 г
 = 220 см3 Ј1,066 г/см3 Ј0,08 = 18,76 г
= 220 см3 Ј1,066 г/см3 Ј0,08 = 18,76 г
ќтсюда m (CaCl2Ј 6H2O) = 
и m (H2O) = 234,52 г Ц 37,01 г = 197,51 г
ќтвет: дл€ приготовлени€ раствора необходимо 37,01 г CaCl2Ј 6H2O и 197,51 см3 H2O.
в) —оставим два балансовых уравнени€:
1) по массе раствора:
m (CaCl2) + m1 = mр-ра или
m (CaCl2) + V1r1 = Vr
2) по массе растворенного вещества:
m (CaCl2) + m (CaCl2/исх.р-р) = m (CaCl2/конечн.р-р)
или m(CaCl2) + V1Ј r1Ј w1 = VЈrЈw
ѕодставл€ем данные из услови€ задачи:
m (CaCl2) + V1 Ј1,015 г/см3 = 220 см3 Ј1,066 г/см3 = 234,52 г
m (CaCl2) + V1Ј 1,015 г/см3 Ј0,02 = 220 см3 Ј1,066 г/см3 Ј0,08 =18,76 г
–еша€ систему двух уравнений методом вычитани€ из первого уравнени€ второго, получаем:
V1Ј 1,015 г/см3 Ј(1-0,02) = 234,52 г -18,76 г = 215,76 г
и V1 = 
m (CaCl2) = 234,52 г -216,91 см3 Ј1,015 г/см3 = 14,35 г
ќтвет: дл€ приготовлени€ раствора нужно 14,35 г CaCl2 и 216,91 см3 исходного раствора с w (CaCl2)= 2 %
г) —оставим два балансовых уравнени€:
1) по массе раствора V1 Јr1 + m (H2O) = VЈr
2) по массе растворенного вещества V1Јr1Јw1 = VЈrЈw
получим систему: V1Јr1 + m (H2O) = VЈr
V1Јr1Јw1 = VЈrЈw
подставл€ем данные из услови€ задачи:
V1 Ј 1,228 г/см3 + m(H2O) = 220 см3 Ј1,066 г/ см3 =234,52 г
V1 Ј1,228 г/см3 Ј0,25 = 220 см3 Ј1,066 г/см3 Ј0,08=18,76 г
ќтсюда  и
и
m (H2O)= 234,52 г Ц (61,11 см3 ∙ 1,228 г/см3) =159,48 г
ќтвет: дл€ приготовлени€ раствора необходимо 61,11 см3 исходного раствора с w(CaCl2)= 25 % и 159,48 см3 H2O
д) —оставим два балансовых уравнени€:
1)-по массе раствора V1r1 + V2r2= VЈr
2)-по массе растворенного вещества V1 Јr1 Јw1+ V2 Јr2 Јw2 = VЈrЈw
ѕодставл€ем данные из услови€ задачи:
V1 Ј1,228 г/см3 +V2 Ј1,015 г/см3 = 220 см3 Ј1,066 г/см3 =234,52 г
V1 Ј1,228 г/см3 Ј0,25 + V2 Ј1,015 г/см3 Ј0,02 = 220 см3 Ј1,066 г/см3 Ј0,08=18,76 г
–еша€ систему двух уравнений с двум€ неизвестными, находим V1 =49,81 см3 и V2 =170,79 см3.
ќтвет: дл€ приготовлени€ смешанного раствора необходимо 49,81 см3 раствора с w (CaCl2) =25 % и 170,79 см3 раствора с w (CaCl2) =2 %.
е) ѕри выпаривании раствора масса растворенного вещества не измен€етс€, а уменьшаетс€ лишь масса летучего растворител€ - воды. —оставим балансовое уравнение по массе растворенного вещества и определим объем исходного раствора, который необходимо вз€ть дл€ выпаривани€:
m (CaCl2/до выпаривани€)=m (CaCl2/после выпаривани€)
или V1 Јr1 Јw1 = VЈrЈw, откуда 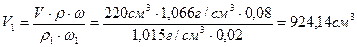
ќпределим массу воды, которую необходимо выпарить:
m(H2O)=m(исх.р-р) Ц m(конечн.р-р)=V1Јr1-VЈr = 924,14 см3 Ј1,015 г/см3 Ц 220 см3 Ј1,066 г/см3 =703,48 г.
ќтвет: нужно вз€ть 924,14 см3 раствора с w (CaCl2) =2 % и 703,48 см3H2O.
ѕример 2. акие объемы газообразного аммиака (н.у.) и его раствора с w (NH3) =3 % и плотностью r = 0,985 г/см3 необходимо вз€ть дл€ приготовлени€ 100 см3 нашатырного спирта (раствор с w (NH3) =10 % и r = 0,957 г/см3).
–ешение.
«апишем два уравнени€ материального баланса на основе двух составных частей смеси:
| I | II | III(конечный раствор))) |
| NH3(газ) V(NH3)=? (н.у.) | –аствор аммиака w2(NH3)= 3 % r2 = 0,985 г/см3 V2=? | –аствор аммиака V (NH3) = 100 см3 w(NH3) =10 % r = 0,957 г/см3 |
—оставл€ем уравнени€ материального баланса
|
|
|
1) по массе смеси: 
2) по массе аммиака  , где Vm = 22,4 дм3 /моль.
, где Vm = 22,4 дм3 /моль.
ѕодставив данные задачи, получим систему двух уравнений:


–еша€ систему из двух уравнений, находим необходимые объемы 3 % раствора аммиака (V2) и объема аммиака (газа): V(NH3) =8,9 дм3; V2 = 90,3 см3
ќтвет: дл€ приготовлени€ 100 см3 нашатырного спирта потребуетс€ 90,3 см3 3% -ного раствора аммиака (r = 0,985 г/см3) и 8,9 дм3 (н.у) NH3
ѕример 3. акой объем раствора сол€ной кислоты с w (HCl) = 20 % и r = 1,098 г/см3 необходим дл€ приготовлени€ одного литра 2ћ раствора?
–ешение.
»сходный раствор HCl €вл€етс€ более концентрированным, чем 2 ћ раствор и приготовление последнего св€зано с разбавлением первого раствора водой. ѕоскольку при добавлении чистого растворител€ содержание чистого вещества не мен€етс€, то вз€тый дл€ приготовлени€ объем Vр-ра с w (HCl) =20 % должен содержать такую же массу HCl, что и конечный разбавленный раствор:
m исх.р-р (HCl) =m конечн.р-р (HCl) или VЈrЈw (HCl) =c(HCl)ЈVЈM(HCl) или
V Ј1,098 г/см3 Ј 0,20 = 2 моль/дм3 Ј1 дм3 Ј36,5 г/моль, тогда V = 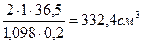 .
.
ќтвет: объем раствора кислоты необходимый дл€ приготовлени€ 1 дм3 2 ћ раствора HCl, равен 332,4 см3.
ћетодика приготовлени€ 2 ћ раствора: в мерную колбу на 1 дм3 внос€т 332,4 см3 исходного раствора с w (HCl) =20 % и затем разбавл€ют дистиллированной водой до метки, до общего объема в 1 дм3.
≈сли при смешивании растворов некоторых веществ протекают химические реакции, то количества реагирующих веществ наход€т через заданные концентрации растворов. ¬ этих случа€х важно безукоризненно знать формулировку каждого способа выражени€ концентрации раствора, а также формулы св€зи между концентраци€ми, количеством растворенного вещества и его массой. роме того, следует обратить внимание на правильное определение качественного и количественного состава раствора после окончани€ химической реакции, а также правильное вычисление его массы:
1) количества n [ моль ]продуктов реакции определ€ет исходное вещество, вз€тое в недостатке, а исходное вещество, вз€тое в избытке, всегда присутствует в конечной смеси веществ;
2) масса конечной смеси равна сумме масс составл€ющих ее исходных растворов и чистых веществ, из которой необходимо вычесть массы всех газообразных и малорастворимых продуктов реакции, которые покидают раствор (образование осадка; выделение газа);
3) объем конечной смеси приближенно равен сумме объемов исходных растворов, а при растворении твердых и газообразных веществ конечный объем раствора считаетс€ равным объему растворител€.
ƒл€ достаточно разбавленных растворов можно прин€ть, что rр-р @ r(H2O) =1 г/см3.
ѕример 4. ¬ 250 г раствора фосфорной кислоты с w (H3PO4) = 9,8 % растворили при нагревании 14,2 г оксида фосфора (V). ќпределить массовую долю растворенного вещества в конечном растворе.
–ешение.
1) ѕри растворении P2O5 в растворе фосфорной кислоты образуетс€ дополнительное ее количество в результате химической реакции:
P2O5 + 3H2O (растворитель) Ѓ 2H3PO4(р-р),
поэтому масса кислоты в конечном растворе равна:
m(H3PO4)=m исх.р-р (H3PO4) + m(H3PO4).
–ассчитаем каждое слагаемое в отдельности:
m исх.р-р (H3PO4) =mр-р Јw (H3PO4) =250 г Ј 0,098 = 24,5 г
m(H3PO4/пореакции)=n(H3PO4)
ћ(H3PO4)= 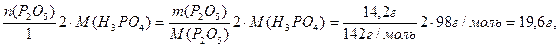
тогда mобщ(H3PO4) =24,5 г + 19,5 г = 44,0 г
2) ћасса конечного раствора кислоты равна:
m(конечн.р-р)=m(исх.р-р) + m(P2O5) =250 + 14,2 = 264,2 г
и w конечн.р-р (H3PO4))=  или 16,69 %
или 16,69 %
ќтвет: массова€ дол€ ортофосфорной кислоты в конечном растворе составила 16,69 %.






