‘ормулы дл€ вычислени€ рЌ растворов слабых оснований выведем на пример NH4OH (NH3 Ј H2O)
NH4OH D NH  +OH-
+OH-
Kосн = 
–ассужда€ аналогично предыдущему примеру, получим: [NH  ]=[OH-]; [NH4OH] ≈ Cосн
]=[OH-]; [NH4OH] ≈ Cосн
осн=  [OH-] =
[OH-] = 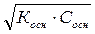
[H+] =  или [H+] =
или [H+] = 
pH = 14- 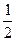 pKосн +
pKосн + 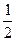 lgCосн
lgCосн
Ѕуферные растворы
Ѕуферные системы (или буферные растворы) Ц это растворы, способные сохран€ть приблизительно посто€нное значение рЌ при добавлении к ним небольших количеств сильных кислот или сильных оснований, или их разбавлений.
Ѕуферные растворы могут содержать либо одно индивидуальное вещество, либо смесь веществ.
„тобы в буферном растворе поддерживалось заданное посто€нное значение рЌ, его готов€т, смешива€ рассчитанные количества компонентов, из которых состоит буферна€ смесь.
„асто используют буферные растворы, содержащие смесь слабой кислоты и ее соли (например, формиатный буфер Ц смесь муравьиной кислоты и формиата натри€ HCOOH +HCOONa, ацетатный буфер Ц смесь уксусной кислоты и ацетата натри€) или смесь слабого основани€ и его соли (например, аммиачный буфер Ц NH4OH и NH4Cl).
–ассмотрим подробнее эти два типа буферных растворов.
1. Ѕуферна€ система, содержаща€ слабую кислоту и ее соль, например ацетатный раствор: —Ќ3—ќќЌ и —Ќ3—ќќNa. Ѕуферное действие ацетатной смеси заключаетс€ в следующем. ≈сли в буферную смесь прибавл€ют небольшой объем сильной кислоты, то ионы водорода этой кислоты, которые могли бы привести к изменению рЌ раствора, будут св€зыватьс€ ацетатными ионами в слабо диссоциирующую уксусную кислоту:
H+ +CH3COO- = CH3COOH
или
HCl+CH3COONa=CH3COOH+H2O.
≈сли к этому же буферному раствору прибавить небольшое количество щелочи, то гидроксид Ц ионы щелочи будут св€зыватьс€ уксусной кислотой
OH- +CH3COOH = CH3COO- +H2O или
NaOH+CH3COOH = CH3COONa+H2O
» в том, и в другом случае баланс ионов водорода в растворе практически не нарушаетс€ и рЌ раствора сохран€етс€ посто€нным.
–ассчитаем величину рЌ в ацетатном буферном растворе:
—Ќ3—ќќNa = CH3COO- +Na+
CH3COOH D CH3COO- +H+
Kк-ты= 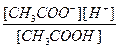
Ќо —Ќ3—ќќЌ Ц очень слаба€ кислота и присутствует в растворе исключительно в виде недиссоциированных молекул, поэтому можно прин€ть [CH3COOH] ≈ Cк-ты. —оль —Ќ3—ќќNa диссоциирована в растворе практически полностью, следовательно, почти все имеющиес€ в растворе ацетат-анионы происход€т от диссоциации соли, тогда [CH3COO-] ≈ Cсоли. ”читыва€ сказанное, приведенное выше выражение дл€ диссоциации принимает вид
к-ты = 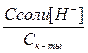
[H+] = Kк-ты 
рЌ=р к-ты -lg 
Ѕуферна€ смесь, содержаща€ слабое основание и его соль, например аммиачный (аммонийный) буфер NH4OH и NH4Cl.
≈сли к аммиачной буферной смеси прибавить небольшое количество сильной кислоты (предположим ЌCl), то ионы Ќ+ этой кислоты св€зываютс€ гидроксидом аммони€
H++NH4OH=NH  +H2O
+H2O
HCl+NH4OH=NH4Cl+H2O
¬ итоге рЌ буферной смеси практически не измен€етс€.
≈сли к аммиачному буферу добавить небольшое количество щелочи ( ќЌ или NaOH), то гидроксильные группы этой щелочи св€зываютс€ катионами аммони€ соли с образованием практически не диссоциирующего в данных услови€х гидроксида аммони€.
|
|
|
NH  +OH-=NH4OH
+OH-=NH4OH
NH4Cl+KOH=NH4OH+KCl
ѕоэтому рЌ раствора и в данном случае практически не мен€етс€.
Ќайдем величину рЌ аммиачного буфера.
NH4Cl=NH4Cl
NH4OH D NH  +OH-
+OH-
Kосн = 
ѕри незначительной степени ионизации NH4OH и практически полной NH4Cl равновесна€ концентраци€ NH4OH будет практически равна его исходной концентрации, а равновесна€ концентраци€ катионов NH  приближенно равна исходной концентрации хлорида аммони€, т.е. [NH4OH]≈Cосн, а [NH
приближенно равна исходной концентрации хлорида аммони€, т.е. [NH4OH]≈Cосн, а [NH  ]≈Cсоли
]≈Cсоли
осн= 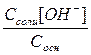
[OH-]=Kосн 
рќЌ=р осн Цlg 
¬с€ка€ буферна€ смесь сохран€ет практически посто€нным рЌ лишь до прибавлени€ некоторого определенного количества кислоты или щелочи, т.е. обладает определенной буферной емкостью.
Ѕуферна€ емкость раствора тем больше, чем выше концентрации компонентов буферной смеси.
¬ качественном и количественном анализах буферные системы используют тогда, когда необходимо поддерживать посто€нное значение рЌ среды. Ќапример, при комплексонометрическом определении некоторых металлов примен€ют аммиачную буферную смесь.
Ѕуферные системы играют большую роль в регулировании жизнеде€тельности организма, в котором должно сохран€тьс€ посто€нство рЌ крови, лимфы и других жидкостей.






