¬ода Ц слабый электролит, поэтому за константу химического равновеси€ можно прин€ть концентрационную константу, выраженную через равновесные концентрации участников реакции:
= 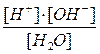
ѕри посто€нной температуре посто€нна и константа химического равновеси€. онцентраци€ чистой воды также посто€нна и равна 55,55 моль/дм3. ѕеренесем посто€нные величины в одну сторону:
[H2O] = [H+] [OH-]
K [H2O] = const K[H2O] =Kw
Kw = [H+] [OH-]
¬еличина Kw называетс€ ионным произведением воды. »з уравнени€ диссоциации воды видно, что [H+] =[OH-] —тепень диссоциации воды весьма мала. ѕри 22 0— в 1дм3 воды распадаетс€ на ионы лишь 10-7мол€ воды (установлено физическими методами следовательно [H+]=[OH-] = 10-7 моль/л.) “огда Kw = [H+] [OH-] =10-14
—мысл этого уравнени€ заключаетс€ в следующем: как бы ни мен€лись концентрации ионов Ќ+ или ќЌ-, их произведение во вс€ком водном растворе сохран€ет приблизительно посто€нное значение, равное 10-14 при 22 0—.
¬с€кий водный раствор, независимо от того, какова его реакци€, должен содержать как ионы Ќ+, так и ионы ќЌ-. ѕоскольку концентрации их св€заны обратно пропорциональной зависимостью, реакцию любого раствора можно охарактеризовать количественно, указав в нем концентрацию ионов Ќ+:
кисла€ среда [H+]>[OH-]>10-7 моль/л
нейтральна€ среда [H+] = [OH-] = 10-7 моль/л
щелочна€ среда [H+]<[OH-]< 107 моль/л
“ак как числовые значени€ Kw, а также [H+] и [OH-] очень маленькие, то вместо них прин€то использовать дес€тичный логарифм этих величин с противоположным знаком, при этом Цlg[H+] обозначаетс€ рЌ и называетс€ водородным показателем; Цlg[ќH-] ЦрќЌ Ц гидроксильным показателем.
¬ кислой среде рЌ<7
¬ нейтральной среде рЌ =7
¬ щелочной среде рЌ>7
≈сли уравнение [H+][OH-]=10-14 прологарифмировать, а затем перен€ть у логарифмов знаки на обратные, то получим
рЌ+рќЌ=14
–асчет рЌ растворов кислот и оснований.
–астворы сильных кислот
ак известно, сильные кислоты практически полностью диссоциируют в растворах, например:
HCl=H+ +Cl-
рH=-lg[H+]
[H+]=α Ј n ЈCк-ты,
где α Ц степень диссоциации кислоты, n Ц число ионов Ќ+, получающихс€ в результате диссоциации, — Ц концентраци€ кислоты, моль/л α ≈1; n=1, тогда [H+] ≈ Cк-ты, а рЌ=-lgCк-ты.
–астворы сильных оснований, например NaOH.
–ассужда€ аналогично, получаем
NaOH=Na+ +OH-
[OH-]= α Ј n Ј Cосн
n=1, α ≈1, [OH-] ≈ Cосн
-lg[OH-] =-lgCосн
[H+] = 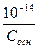 → рЌ =14+lgCосн
→ рЌ =14+lgCосн
–астворы слабых кислот
¬ыведем формулу дл€ вычислени€ рЌ растворов слабых кислот на примере уксусной кислоты
CH3COOH D CH3COO- + H+
Kк-ты= 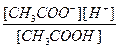
≈сли раствор кислоты не слишком разбавлен, то почти вс€ она присутствует в виде недиссоциированных молекул, следовательно [CH3COOH] ≈ const. »з уравнени€ диссоциации кислоты видно, что [H+] = [CH3COO-], тогда
к-ты= 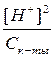
[H+]2 = Kк-ты Ј —к-ты,
[H+] = 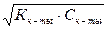
pH = 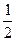 pKк-ты -
pKк-ты - 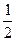 lgCк-ты,
lgCк-ты,
где р = -lgK






