61. ”равнение состо€ни€ газа выведено ƒ.». ћенделеевым и лайпероном. ќно записываетс€: 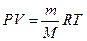 , где – - давление, при котором находитс€ газ, V - объем газа, m - масса газа, ћ - молекул€рна€ масса газа, R - мол€рна€ газова€ посто€нна€, “ - температура системы. ”равнение также называетс€ уравнением ћенделеева- лайперона. ѕример: найти объем 3-х моль газа, наход€щегос€ при 106.8 кѕа и температуре 400 :
, где – - давление, при котором находитс€ газ, V - объем газа, m - масса газа, ћ - молекул€рна€ масса газа, R - мол€рна€ газова€ посто€нна€, “ - температура системы. ”равнение также называетс€ уравнением ћенделеева- лайперона. ѕример: найти объем 3-х моль газа, наход€щегос€ при 106.8 кѕа и температуре 400 : 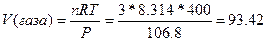 литра.
литра.
62. 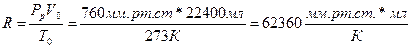
63. а) 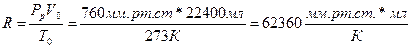
б) 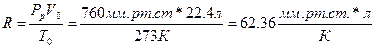
в) 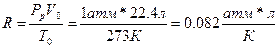
64. √аз называетс€ идеальным, если газ подчин€етс€ законам лайперона-ћенделеева и –аул€. ’арактеристика реального газа близка к идеальному, если форма его молекул близка к идеальному шару, если отсутствует взаимодействие между молекулами и столкновени€ молекул можно прин€ть за упругое соударение.
65. 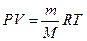 , следовательно,
, следовательно, 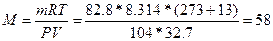 г/моль.
г/моль.
66. 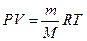 , следовательно,
, следовательно, 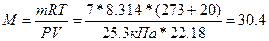 г/моль.
г/моль.
67. 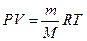 , следовательно,
, следовательно, 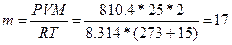 г.
г.
68. 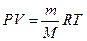 , =>
, => 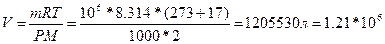 литров.
литров.
69. 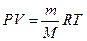 , следовательно,
, следовательно, 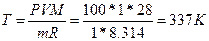
70. 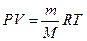 , =>
, => 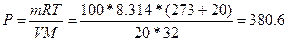 кѕа
кѕа
71. ”равнение лайперона записываетс€: 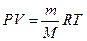 , где – - давление, при котором находитс€ газ, V - объем газа, m - масса газа, ћ - молекул€рна€ масса газа, R - мол€рна€ газова€ посто€нна€, “ - температура системы. ќно используетс€ при нахождении неизвестного параметра состо€ни€ газа (давлени€, массы, температуры, мол€рной массы, объема) по известным параметрам, вход€щим в уравнение. ѕример: найти объем 3-х моль газа, наход€щегос€ при 106.8 кѕа и температуре 400 :
, где – - давление, при котором находитс€ газ, V - объем газа, m - масса газа, ћ - молекул€рна€ масса газа, R - мол€рна€ газова€ посто€нна€, “ - температура системы. ќно используетс€ при нахождении неизвестного параметра состо€ни€ газа (давлени€, массы, температуры, мол€рной массы, объема) по известным параметрам, вход€щим в уравнение. ѕример: найти объем 3-х моль газа, наход€щегос€ при 106.8 кѕа и температуре 400 : 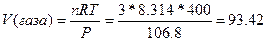 литра.
литра.
72. Ќормальными услови€ми дл€ газов €вл€ютс€ 298 , 101,3 кѕа. 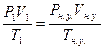 =>
=> 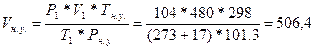 литра
литра
73. Ќормальными услови€ми дл€ газов €вл€ютс€ 298 , 101,3 кѕа. 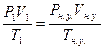 =>
=> 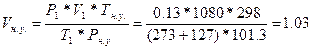 литра.
литра.
74. ѕарциальное давление газа пропорционально объемной доле газа в смеси газов. –Т(N2) = –(общ)*w%(N2) / 100% = 101325*78.1 / 100 = 79135 ѕа. –Т(ќ2) = –(общ)*w%(ќ2) / 100% = 101325*20.9 / 100 = 21177 ѕа. –Т(Ar) = –(общ)*w%(Ar) / 100% = 101325*0.93 / 100 = 942 ѕа.. –Т(—ќ2) = –(общ)*w%(—ќ2) / 100% = 101325*0.03 / 100 = 30.4 ѕа.
75. ѕарциальное давление газа пропорционально объемной доле газа в смеси газов: w%(NO) =P(NO) * 100% / P(общ) = 39990*100 / (39990 + 66630) = 37,5%; w%(NO2) =P(NO2) * 100% / P(общ) = 66630*100 / (39990 + 66630) = 62,5%;
76.
| ƒано V(CH4) = 0.03 м3 –(—Ќ4) = 96.0 кѕа V(H2) = 0.04 м3 –(Ќ2) = 84.0 кѕа V(Cќ) = 0.01 м3 –(—ќ) = 108.8 кѕа | Ќайдем количество каждого вещества из закона лайперона-ћенделеева: n = PV/RT n(CH4) = 96*30/(8.31*298) = 1.163 моль. ƒол€ газа х1 = 1.163*100%/2.959 = 39,3% n(H2) = 84*40/(8.31*298) = 1.357 моль. ƒол€ газа х1 = 1.357*100%/2.959 = 45,9% n(Cќ) = 108.8*10/(8.31*298) = 0.439 моль. ƒол€ газа х1 = 0.439*100%/2.959 = 14,8% Vобщ = 30 + 40 + 10 = 80 литров, n(общ) = 1.163+1.357+0.439 = 2.959 моль, следовательно, –общ = nRT/V = 2.959*8.31*298/80 = 91.6 кѕа ѕарциальные давлени€: ѕ(—Ќ4) = 91.6*0.393 = 36 кѕа, ѕ(Ќ2) = 91.6*0.459 = 42 кѕа, ѕ(—ќ) = 91,6*0.148 = 13,6 кѕа |
77. V1(N2) = 120мл = 0.120 л; “ = 200— = 293 ; –(общ) = 100 кѕа, –(Ќ2ќ) = 2.3 кѕа. Vн.у.(N2) =?. Ќайдем парциальное давление азота: –1(N2)= –(общ) - –(Ќ2ќ) = 100 - 2.3 = 97.3 кѕа. 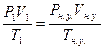 =>
=> 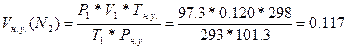 литра.
литра.
|
|
|
78. V1(Ќ2) = 250мл = 0.250 л; “ = 260— = 299 ; –(общ) = 98.7 кѕа, –(Ќ2ќ) = 3.4 кѕа. Vн.у.(Ќ2) =?. Ќайдем парциальное давление водорода: –1(Ќ2)= –(общ) - –(Ќ2ќ) = 98.7 - 3.4 = 95.3 кѕа. 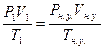 =>
=> 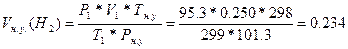 литра.
литра.
79. «акон јвогадро: ¬ равных объемах различных газов при одинаковых температуре и давлении содержитс€ одинаковое число молекул.
—ледствие 1. ќбъемы, занимаемые 1 моль газа, должны быть одинаковыми дл€ всех газов.
—ледствие 2. ќдин моль любого газа содержит 6,02*1023 соответствующих молекул.
„исло Na = 6.02*1023 носит название посто€нной јвогадро и выведено с использованием его закона. »з закона јвогадро следует, что два газа одинаковых объемов, хот€ и содержат равное число молекул, имеют неодинаковые массы: масса одного газ во столько же раз больше массы другого газа, во сколько раз относительна€ молекул€рна€ масса первого больше, чем относительна€ молекул€рна€ масса второго. «акон јвогадро имел фундаментальное значение в истории развити€ атомно-молекул€рного учени€.
80. «акон јвогадро: ¬ равных объемах различных газов при одинаковых температуре и давлении содержитс€ одинаковое число молекул. m(O2) = 8.0 г. n(O2) = m/M = 8 / 32 = 0.25 моль. V(O2) = n*Vм = 0.25 * 22.4 = 5.6 литров.
81. V(H2) = 280 литров. n(H2) = V/Vм = 280 / 22.4 = 12.5 моль. m(Ќ2) = n(H2)* M(Ќ2) = 12.5*2 = 25 грамм.
82. V(газа) = 600 мл. n(газа) = V/Vм = 0.6 / 22.4 = 0.0268 моль. M(газа) = m(газа) / n(газа) = 1.714 / 0.0268 = 64 грамм/моль.
83. n(—ќ2) = 10 моль. V(—O2) = n*Vм = 10 * 22.4 = 224 литра. m(—ќ2) = n(—ќ2)* M(—ќ2) = 10*44 = 440 грамм.
84. V(газа) = 2 л. n(газа) = V/Vм = 2 / 22.4 = 0.089 моль. а) ћол€рна€ масса газа: M(газа) = m(газа) / n(газа) = 2.5 / 0.089 = 28 грамм/моль. б) ћолекул€рна€ масса газа = 28. в) абсолютна€ масса одной молекулы m = 28 / 6,02*1023 = 4,65 *10-23 грамм.
85. а) ƒаны равные количества азота и водорода: n(H2) = n(N2). —огласно закону јвогадро, Ђв равных объемах различных газов при одинаковых температуре и давлении содержитс€ одинаковое число молекулї, т.е. отношение объемов азота и водорода равно 1.
б) ƒаны равные массы азота и водорода: m(H2) = m(N2). => V(H2) = n(H2)/Vм = = 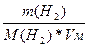 =>
=> 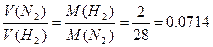
86. √аз называетс€ идеальным, если газ подчин€етс€ законам лайперона-ћенделеева и –аул€. ’арактеристика реального газа близка к идеальному, если форма его молекул близка к идеальному шару, если отсутствует взаимодействие между молекулами и столкновени€ молекул можно прин€ть за упругое соударение. ћолекулы водорода по свойствам более близки к идеальному газу, чем молекулы азота. ѕоэтому мол€рный объем водорода ближе к идеальному значению, чем мол€рный объем азота.
87. ќтносительна€ плотность газа вещества ј по веществу B - отношение относительной молекул€рной массы ј к относительной молекул€рной массе вещества B. DB(A)=M(A)/MB). Dвоздуху(Cl2) = M(Cl2)/M(воздуха) = 71/29 = 2.45; Dкислороду(—2Ќ4) = M(C2Ќ4)/M(ќ2) = 28/32 = 0.875.
88. Dвоздуху(јцетона)=2. DB(A)=M(A)/MB), следовательно, ћ(ацетона) = Dвоздуху*ћ(воздуха) = 2*29 = 58 г/моль.
89. Dкислороду(Sx)=8. DB(A)=M(A)/MB), следовательно, ћ(Sx)= Dкислороду*ћ(кислорода) = 8*32 = 256 г/моль. х = ћ(Sx)/ ћ(S) = 256/32 = 8 атомов.
90. Dводороду(N2+O2)=15. DB(A)=M(A)/MB). —редн€€ молекул€рна€ масса смеси азота и кислорода равна ћ(N2+O2) = Dводороду*ћ(водорода) = 15*2 = 30 г/моль. ѕусть х и у - соответственно объемные доли азота и кислорода в смеси. —оставим систему:
|
|
|
х*ћ(N2) + у*ћ(O2) = 30
х + у = 1, следовательно у = 1 - х
х*ћ(N2) + (1 - х)*ћ(O2) = 30 => 28x + 32 - 32x = 30 => x = 0.5 => y = 1 - 0.5 = 0.5
91. ¬алентностью данного элемента называетс€ число атомов соедин€ющегос€ с ним другого элемента. элементам переменной валентности относ€тс€: азот: B=1 - N2O, B=2 - NO, B=3 - N2O3; железо: ¬=2 - FeO, B=3 Fe2O3, и многие другие элементы.
92. ¬алентностью данного элемента называетс€ число атомов соедин€ющегос€ с ним другого элемента, т.е. валентность - это число св€зей с другими элементами, которые образует данный элемент. ѕосто€нна€ валентность = 1: , Li, Na, Rb - KCl, LiF, NaOH, RbF. ѕосто€нна€ валентность = 2: Ca, Mg, Be - CaS, Mg(OH)2, BeCl2, ѕосто€нна€ валентность = 3: Al, Ga, In - AlPO4, Ga(OH)3, InCl3, элементам переменной валентности относ€тс€: азот: B=1 - N2O, B=2 - NO, B=3 - N2O3; железо: ¬=2 - FeO, B=3 Fe2O3, и многие другие элементы.
93. ¬алентностью данного элемента называетс€ число атомов соедин€ющегос€ с ним другого элемента, т.е. валентность - это число св€зей с другими элементами, которые образует данный элемент. —оответственно, максимальна€ валентность - это максимальное число св€зей, которые может образовать элемент в соединении. ћаксимальна€ валентность: P = 5 - H3PO4, Cl = 7 - HClO4, S = 6 - SO3, Xe = 8 - XeF8.
94. ћаксимальна€ валентность: Sn =4 - H2SnO3, Sb =5 - SbCl5, Se =6 - SeO3, Br =7 - HBrO4.
95. ћаксимальна€ валентность: V =5 - HVO3, Cr =6 - K2CrO4, Mn =7 - KMnO4, Ru =8 - RuO4.
96. «начени€ валентности различаютс€ на 2 у: серы - SO2 / SO3, хлора - HClO / HClO2, талли€ - TlCl / TlCl3.
97. «начени€ валентности различаютс€ на 1 у: меди - Cu2O / CuO, железа - FeO / Fe2O3, титана - Ti2O3 / TiO2.
98.
| —оединение | H2S | SO3 | H2SO3 | SO2 | H2SO4 | CS2 |
| ¬алентность серы |
99.
| —оединение | NH3 | N2O | NO | N2O3 | HNO2 | NO2 | N2O5 | HNO3 | NH4NO3 | NH4NO2 | N2H4 | Na3N |
| ¬алентность азота | 3,5 | 3,3 |
100.
| —оединение | NaCl | CaCl2 | Cl2O | HClO | HClO2 | KClO3 | Cl2O7 | HClO4 |
| ¬алентность хлора |
101. ѕо периоду максимальна€ валентность химических элементов увеличиваетс€ в св€зи с добавлением валентных электронов. Si: ¬=4 - SiO2, P: ¬=5 - PCl5, S: ¬=6 - SO3, I: ¬=7 - HIO4.
102. ћаксимальна€ валентность химического элемента совпадает с номером группы в ѕ—. Ge: ¬=4 - GeO2, P: ¬=5 - PCl5, Se: ¬=6 - SeO3, Cl: ¬=7 - HClO4.
103. ћаксимальна€ валентность химического элемента совпадает с номером группы в периодической системе, поэтому по периоду с увеличением атомного номера максимальна€ валентность увеличиваетс€. ѕо группе, в св€зи с уменьшением прит€жени€ €дром валентных (внешних) электронов максимальна€ валентность с увеличением атомного номера уменьшаетс€. ћаксимальна€ валентность Sn =4 - H2SnO3, Sb =5 - SbCl5, “e =6 - “eO3, Br =7 - HBrO4, Xe =8 - XeF8.
104. ћаксимальна€ валентность химического элемента совпадает с номером группы в ѕ—. “ретий период - Na: B=1 - NaCl, Mg: B=2 - MgS, Al: B=3 - AlN, Si: ¬=4 - SiO2, P: ¬=5 - PCl5, S: ¬=6 - SO3, Cl: ¬=7 - HClO4.
105. ћаксимальна€ валентность химического элемента совпадает с номером группы в ѕ—. „етвертый период - K: B=1 - KCl, Ca: B=2 - CaS, Ga: B=3 - GaN, Ge: ¬=4 - GeO2, As: ¬=5 - AsCl5, Se: ¬=6 - SeO3, Br: ¬=7 - HBrO4.
106. Ёто переходные d-,f-металлы. Ќапример, Co, Rh, Tb, Cm: Co(OH)3, RhCl3, TbCl3, CmBr3.
107. AlH3, Al2O3, AlN, Al4C3, Al2S3, AlF3
108. PH3, P2O5, AlN, Na3P, P2S5, PF5
109. ’лориды: BCl3,SiCl4, PCl5, SnCl2, MgCl2, —ульфиды: B2S3, SiS2, P2S5, SnS, MgS.
110. ќксиды азота: I - N2O, II - NO, III - N2O3, IV - NO2, V - N2O5; ћарганца: II - MnO, III - Mn2O3, IV - MnO2, VI - MnO3, VII - Mn2O7; ’рома: II - CrO, III - Cr2O73, IV - CrO2.
111. —труктурна€ формула не отражает реального строени€ молекулы или элементарной кристаллической решетки, однако позвол€ет рассчитать кратность св€зей в молекуле и валентность атомов. Al2O3 - O=Al-O-Al=O,
|
|
|
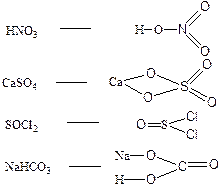
112. —труктурна€ формула позвол€ет рассчитать кратность св€зей в молекуле и валентность атомов.
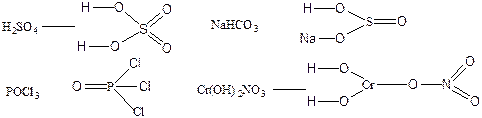
113. —труктурна€ формула позвол€ет рассчитать кратность св€зей в молекуле и валентность атомов.
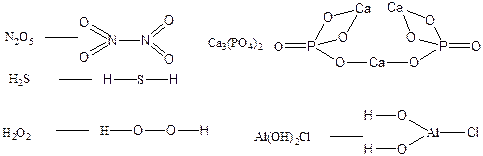
115. ¬алентность элементов совпадает с количеством св€зей между атомами в молекуле:
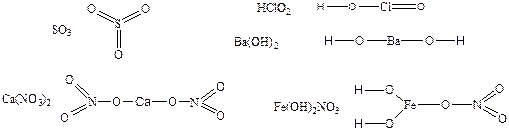
—труктурна€ формула не отражает реального строени€ молекулы или элементарной кристаллической решетки.
116. ‘осфорна€ кислота - трехосновна€, фосфориста€ - двухосновна€, фосфорноватиста€ - одноосновна€ (по числу атомов водорода, св€занных с атомами кислорода).
117. Ќомер группы, в которой элемент расположен, совпадает с валентностью элемента по кислороду. Ќомер группы минус 8 - валентность элемента по водороду. ”глерод (4 группа) - по кислороду ¬=4, по водороду ¬=8-4=4, азот (5 группа) - по кислороду ¬=5, по водороду ¬=8-5=3. сера (6 группа) - по кислороду ¬=6, по водороду ¬=8-6=2, хлор (7 группа) - по кислороду ¬=7, по водороду ¬=8-7=1.
118. ¬ соединени€х с кислородом валентность элемента совпадает с количеством валентных электронов, т.к. кислород прит€гивает электроны, а в соединении с водородом - с числом вакантных мест дл€ электронов на валентном уровне, т.к. водород отдает электроны. —умма вакантных мест и валентных электронов равна восьми, что объ€сн€ет наблюдаемую закономерность.
119. Ёлектронна€ валентность кислорода одинакова, а стехиометрическа€ разна€ в соединени€х: Ќ2ќ (¬э=2, ¬с=2) и Ќ2ќ2 - (¬э=2, ¬с=1).
120. ’лорид аммони€: NH4Cl - электронна€ валентность =3, стехиометрическа€ = 4; перекись водорода: Ќ2ќ2 - электронна€ валентность =2, стехиометрическа€ = 1.






