ѕриклад 1. –озрахувати розчинн≥сть CaSO4 у вод≥, в розчин≥ KNO3 з мол€рною концентрац≥Їю кал≥й н≥трату 0,01 моль/дм 3 ≥ в розчин≥ Mg(NO3)2 з мол€рною концентрац≥Їю магн≥й н≥трату 0,01 моль/дм 3.
ƒано:
c (KNO3) = 0,01 моль/дм 3 ƒP (CaSO4) =2,37∙10Ц5
 c (Mg(NO3)2) = 0,01 моль/дм 3
c (Mg(NO3)2) = 0,01 моль/дм 3
S (CaSO4) Ц?
–озвТ€зуванн€
ѕозначимо розчинн≥сть CaSO4 у вод≥ через х. “ак €к CaSO4 при розчиненн≥ даЇ по одному йону Ca2+ ≥  ,то
,то
[Ca2+] = [  ] = [CaSO4] = x
] = [CaSO4] = x
ƒ–(CaSO4) = [Ca2+]Ј[  ] = x Ј x = 2,37∙10Ц5
] = x Ј x = 2,37∙10Ц5
x 2= 2,37Ј10Ц5; x =  = 4,868Ј10Ц3 моль/дм 3
= 4,868Ј10Ц3 моль/дм 3
«найдемо розчинн≥сть CaSO4 у розчин≥ KNO3 з мол€рною концентрац≥Їю речовини 0,01 моль/дм 3. …онна сила розчину буде визначатис€ концентрац≥Їю ≥ зар€дами вс≥х присутн≥х у розчин≥ йон≥в:
μ = ½([K+]Ј12 + [ 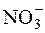 ]Ј12 + [Ca2+]Ј22 + [
]Ј12 + [Ca2+]Ј22 + [  ]Ј22) = ½(0,01Ј12 +
]Ј22) = ½(0,01Ј12 +
+ 0,01Ј12 + 0,004868Ј22 + 0,004868Ј22) = ½(0,01 + 0,01 + 0,0195 +
+ 0,0195) = 0,0295
“ак €к у таблиц≥ немаЇ йонноњ сили розчину, що дор≥внюЇ 0,0295, то величину коеф≥ц≥Їнт≥в активност≥  =
=  знаход€ть методом ≥нтерпол€ц≥њ:
знаход€ть методом ≥нтерпол€ц≥њ:
 =
=  = 0,55Ц0,018 = 0,532 або приблизно 0,53
= 0,55Ц0,018 = 0,532 або приблизно 0,53
ƒ– (CaSO4) = [Ca2+]Ј[  ]∙
]∙  ∙
∙  = x ∙ x ∙0,532 = 2,37∙10Ц5
= x ∙ x ∙0,532 = 2,37∙10Ц5
x 2∙0,532 = 2,37∙10Ц5
x =  = 9,2∙10Ц3 моль/дм 3
= 9,2∙10Ц3 моль/дм 3
«найдемо розчинн≥сть —aSO4 у розчин≥ Mg(NO3)2 з мол€рною концентрац≥Їю речовини 0,01 моль/дм 3.
” цьому випадку йонна сила розчину становить:
μ = ½([Mg2+]∙22 + 2[ 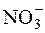 ]Ј12 + [Ca2+]Ј22 + [
]Ј12 + [Ca2+]Ј22 + [  ]Ј22) =
]Ј22) =
= ½(0,01Ј4 + 2Ј0,01 + 0,004868Ј4 + 0,004868Ј4) = 0,0495 ≈ 0,05
ќтже,  =
=  = 0,45.
= 0,45.
ƒ– (CaSO4) = [Ca2+]∙[  ]∙
]∙  ∙
∙  = x ∙ x ∙0,452 = 2,37∙10Ц5
= x ∙ x ∙0,452 = 2,37∙10Ц5
x 2∙0,452 = 2,37∙10Ц5
x =  = 1,082∙10Ц2 моль/дм 3
= 1,082∙10Ц2 моль/дм 3
« цих розрахунк≥в випливаЇ, що розчинн≥сть кальц≥й сульфату в розчинн≥ кал≥й н≥трату (с =0,01 моль/дм 3) приблизно в 1,9 рази б≥льша в≥д розчинност≥ у чист≥й вод≥, а розчинн≥сть CaSO4 у розчин≥ Mg(NO3)2 (с =0,01 моль/дм 3) приблизно в 2,2 рази б≥льша, н≥ж у чист≥й вод≥.
ѕриклад 2. ќбчислити ≥нтервал значень p H розчину дл€ к≥льк≥сного розд≥ленн€ йон≥в Fe3+ ≥ Mg2+ шл€хом осадженн€ њх у форм≥ г≥дроксид≥в.
–озвТ€зуванн€
—початку розраховуЇтьс€ значенн€ p H розчину, при €кому буде дос€гнута повнота посадженн€ йон≥в Fe3+ у вигл€д≥ Fe(OH)3. ритер≥Їм повноти осадженн€ буде [Fe3+ ]£1,0∙10Ц6 моль/дм 3.
¬иход€чи з добутку розчинност≥
ƒ– (Fe(OH)3) = [Fe3+]∙[OHЦ]3 = 6,3∙10Ц38,
знаход€ть концентрац≥ю йон≥в ќЌЦ, при €к≥й буде дос€гнута повнота осадженн€ йон≥в Fe3+:
 моль/дм 3
моль/дм 3
«в≥дси
 моль/дм 3
моль/дм 3
рH = Цlg[H+] = Цlg(2,5∙10Ц4) = 3,6
ќтже, дл€ повноти осадженн€ йон≥в Fe3+ у форм≥ Fe(OH)3 необх≥дно п≥дтримувати в розчинн≥ p H 3,6.
…они Mg2+, будуть залишатис€ в розчинн≥ до тих п≥р, доки не буде дос€гнуто добуток розчинност≥ Mg(OH)2, тобто умовою збереженн€ йон≥в Mg2+ у розчинн≥ буде
[Mg2+]∙[OHЦ]2 < ƒ– (Mg(OH)2); ƒ–(Mg(OH)2) = 6,0∙10Ц10
«в≥дси
[OHЦ]£  £
£  моль/дм 3
моль/дм 3
|
|
|
якщо у вих≥дному розчин≥ [Mg2+]=0,1 моль/дм 3, то умовою невипад≥нн€ в осад Mg(OH)2 буде
[OHЦ]£  моль/дм 3; [OHЦ]£7,8∙10Ц5 моль/дм 3,
моль/дм 3; [OHЦ]£7,8∙10Ц5 моль/дм 3,
а €кщо [Mg2+]=1,0∙10Ц2 моль/дм 3, то
[OHЦ]£  моль/дм 3; [OHЦ]£2,5∙10Ц4 моль/дм 3,
моль/дм 3; [OHЦ]£2,5∙10Ц4 моль/дм 3,
якщо концентрац≥€ йон≥в Mg2+ у вих≥дному розчин≥ становить 0,1 моль/дм 3, то дл€ збереженн€ цих йон≥в у розчин≥ [OHЦ]£7,8∙10Ц5 моль/дм 3, що в≥дпов≥даЇ
[H+]=  =1,3Ј10Ц10 моль/дм 3, або p H£9,9.
=1,3Ј10Ц10 моль/дм 3, або p H£9,9.
“аким чином, €кщо у розчин≥ буде п≥дтримуватис€ значенн€ p H не менше, н≥ж 3,6 ≥ не б≥льше, н≥ж 9,9, то Fe3+ повн≥стю буде в осад≥ у вигл€д≥ Fe(OH)3, а йони Mg2+ повн≥стю залишатьс€ в розчин≥. ÷ю умову можна записати у вигл€д≥ формули:
3,6£ p H£9,9.
ѕриклад 3. який обТЇм розчину аргентум н≥трату з масовою часткою AgNO3 2% необх≥дно вз€ти дл€ осадженн€ йон≥в ClЦ ≥з наважки CaCl2∙6H2O масою 0,4382 г?
ƒано:
m (CaCl2∙6H2O) = 0,4382 г
 w (AgNO3) = 2%
w (AgNO3) = 2%
V (AgNO3(p)) Ц?
–озвТ€зуванн€
ќбчисленн€ виконуютьс€ наближено, тому числа, €к≥ берутьс€ дл€ розрахунк≥в, сл≥д округлити.
¬изначаЇтьс€ маса аргентум н≥трату, необх≥дна дл€ осадженн€ йон≥в ClЦ:

ƒал≥ визначаЇтьс€ обТЇм розчину AgNO3 (w (AgNO3)=2%), у €кому м≥ститьс€ розрахована маса AgNO3. √устину розчину можна прийн€ти за одиницю.

ѕриклад 4. ќбчислити втрату маси ≥ в≥дносну похибку за рахунок розчинност≥ осаду CaC2O4ЈH2O, €кщо до 20 см 3 розчину CaCl2 з мол€рною концентрац≥Їю речовини CaCl2 0,1 моль/дм 3 додали 30 см 3 розчину (NH4)2C2O4 з мол€рною концентрац≥Їю речовини амон≥й оксалату 0,1 моль/дм 3.
ƒано:
V (CaCl2(p)) = 20 см 3 ƒ– (—а—2ќ4) = 2,3∙10Ц9
с (CaCl2) = 0,1 моль/дм 3 ћ (—а—2ќ4∙Ќ2ќ) = 146,12 г/моль
V ((NH4)2C2O4(p)) = 30 см 3
с ((NH4)2C2O4) = 0,1 моль/дм 3
 m (CaC2O4ЈH2O) Ц?
m (CaC2O4ЈH2O) Ц?
η Ц?
–озвТ€зуванн€
ѕ≥сл€ зливанн€ розчин≥в кальц≥й хлориду ≥ амон≥й оксалату обТЇм утвореного розчину становить: 20 см 3+30 см 3=50 см 3. Ќадлишок розчину осаджувача складаЇ 10 см 3. онцентрац≥€ оксалат-≥он≥в у отриманому розчин≥ дор≥внюЇ:
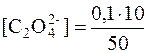 = 0,02 моль/дм 3
= 0,02 моль/дм 3
ѕозначимо через х розчинн≥сть —а—2ќ4∙Ќ2ќ. “од≥ [—а2+]= х моль/дм3, [  ]=(0,02+ х) моль/дм 3. ¬иход€чи ≥з значенн€ ƒ– (—а—2ќ4),
]=(0,02+ х) моль/дм 3. ¬иход€чи ≥з значенн€ ƒ– (—а—2ќ4),
[—а2+]Ј[  ] = х ∙(0,02+ х) = 2,3∙10Ц9
] = х ∙(0,02+ х) = 2,3∙10Ц9
“ак €к х Ђ 0,02, то (0,02+ х) ≈ 0,02. ќтже, х ∙0,02 = 2,3∙10Ц9. «в≥дси:
 = 1,15∙10Ц7 моль/дм 3
= 1,15∙10Ц7 моль/дм 3
–озчинн≥сть —а—2ќ4 дор≥внюЇ 1,15∙10Ц7 моль/дм 3. ¬трати через розчинн≥сть кальц≥й оксалату у розчин≥ обТЇмом 50 см 3 становитимуть:
 = 8,4∙10Ц7 г.
= 8,4∙10Ц7 г.
ћаса осаду —а—2ќ4∙Ќ2ќ, отриманого при зливанн≥ розчин≥в кальц≥й хлориду ≥ амон≥й оксалату становить:
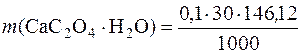 = 0,4383 г.
= 0,4383 г.
“од≥ η =  = 1,9Ј10Ц4%.
= 1,9Ј10Ц4%.
ѕриклад 5. ќбчислити масову частку втрат магн≥й г≥дроксиду за рахунок розчинност≥ осаду Mg(OH)2 масою 0,2 г при промиванн≥ водою обТЇмом 250 см 3. ¬изначити концентрац≥ю амон≥аку у промивн≥й р≥дин≥, щоб втрати при промиванн≥ осаду таким же обТЇмом склали не б≥льше 0,1%.
ƒано:
m (Mg(OH)2) = 0,2 г ƒ– (Mg(OH)2) = 6,0Ј10Ц10
V (H2O) = 250 см 3 M (Mg(OH)2) = 58,32 г/моль
 V (NH3(р)) = 250 см 3 Kд (NH3ЈH2O) = 1,76Ј10Ц5
V (NH3(р)) = 250 см 3 Kд (NH3ЈH2O) = 1,76Ј10Ц5
w (Mg(OH)2) Ц?
с (NH3(p)) Ц?
–озвТ€зуванн€
¬иход€чи з добутку розчинност≥ Mg(OH)2 визначають концентрац≥ю йон≥в Mg2+ ≥ р≥вну њй концентрац≥ю Mg(OH)2 у водному насиченому розчин≥.
ƒ– (Mg(OH)2) = [Mg2+]Ј[OHЦ]2 = 6,0∙10Ц10.
|
|
|
ѕозначимо через х моль/дм 3 розчинн≥сть Mg(OH)2. “од≥ [Mg2+] = х моль/дм 3, а [OHЦ] = 2 х моль/дм 3. ѕ≥дставл€ючи ц≥ значенн€ у формулу добутку розчинност≥ Mg(OH)2, маЇмо:
х Ј(2 х)2 = 6,0Ј10Ц10; 4 х 3 = 6,0Ј10Ц10; х 3 =  ;
;
х =  = 5,31Ј10Ц4 моль/дм 3.
= 5,31Ј10Ц4 моль/дм 3.
ќтже, втрати при промиванн≥ осаду Mg(OH)2 водою за рахунок розчинност≥ будуть складати:
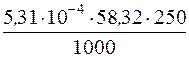 = 7,74Ј10Ц3 г Mg(OH)2,
= 7,74Ј10Ц3 г Mg(OH)2,
або  = 3,87%.
= 3,87%.
якщо промивати осад Mg(OH)2 розчином амон≥аку, розчинн≥сть магн≥й г≥дроксиду знижуЇтьс€ внасл≥док введенн€ однойменних з осадом йон≥в ќЌЦ. «наючи, що втрати Mg(OH)2 при промиванн≥ ц≥Їю промивною р≥диною не повинн≥ перевищувати 0,1%, знаходимо розчинн≥сть Mg(OH)2 в моль/дм 3:
 = 1,37Ј10Ц5 моль/дм 3.
= 1,37Ј10Ц5 моль/дм 3.
ќтже, [Mg2+] = 1,37Ј10Ц5 моль/дм 3.
¬икористовуючи значенн€ добутку розчинност≥ Mg(OH)2, визначаЇмо р≥вноважну концентрац≥ю ќЌЦ-≥он≥в:
6,0Ј10Ц10 = [Mg2+]Ј[OHЦ]2 = 1,37Ј10Ц5Ј[OHЦ]2
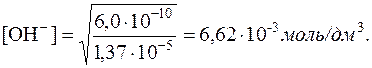
Ќа основ≥ константи дисоц≥ац≥њ NH3ЈH2O

розраховуЇмо концентрац≥ю NH3 у розчин≥, у €кому [OHЦ] =
6,62Ј10Ц3 моль/дм 3. “ак €к у водному розчин≥ амон≥аку [  ]=[OHЦ], то з константи дисоц≥ац≥њ виходить:
]=[OHЦ], то з константи дисоц≥ац≥њ виходить:

ѕриклад 6. ѕ≥сл€ в≥дпов≥дноњ обробки м≥нералу, що м≥стить —ульфур, вз€того масою 2,6248 г було отримано 0,3248 г BaSO4. –озрахувати масову частку —ульфуру у досл≥джуваному м≥нерал≥.
ƒано:
m (м≥нералу) = 2,6248г M (S) = 32,06 г/моль
m (BaSO4) = 0,3248г M (BaSO4) = 233,39 г/моль
 w (S) Ц?
w (S) Ц?
–озвТ€зуванн€
‘актор перерахунку (грав≥метричний множник):
 .
.
“од≥:

ћасова частка —ульфуру у досл≥джуваному м≥нерал≥ складаЇ 1,7%.
І 32. «апитанн€ дл€ самоперев≥рки
1. як≥ вимоги ставл€тьс€ до осаджуваноњ форми у грав≥мет≠ричному анал≥з≥?
2. як≥ вимоги ставл€тьс€ до грав≥метричноњ форми?
3. як впливають на повноту осадженн€ BaSO4 температура розчину, концентрац≥€ ≥ обТЇм розчину осаджувача, на€вн≥сть сторонн≥х електрол≥т≥в?
4. як≥ йони будуть адсорбуватис€ на поверхн≥ осаду на початку осадженн€ BaCl2 розчином Na2SO4 ≥ Na2SO4 розчином BaCl2?
5. як≥ йони будуть адсорбуватис€ на поверхн≥ осаду при додаванн≥ надлишку осаджувача п≥сл€ осадженн€ BaCl2 розчином Na2SO4 ≥ Na2SO4 розчином BaCl2?
6. яку с≥ль Ц Ba(Nќ3)2, BaBr2, BaCl2 чи Ba(ClO4)2 Ц доц≥льно використати €к осаджувач дл€ отриманн€ найб≥льш чистого осаду BaSO4?
7. яку сполуку Ц K2C2O4, Na2C2O4, H2C2O4 чи (NH4)2C2O4 Ц доц≥льно використати €к осаджувач дл€ осадженн€ йон≥в Ca2+?
8. ” €кому випадку втрати при промиванн≥ осаду Fe(OH)3 будуть найменшими: а) при промиванн≥ осаду водою; б) розчином NH4NO3; в) розчином NH4NO3 з NH3?
9. яка сполука найб≥льш придатна €к грав≥метрична форма дл€ к≥льк≥сного визначенн€ Ca2+, Sr2+, Ni2+, Zn2+, Cu2+?
10. як звТ€зано в≥дносне пересиченн€ розчину з числом центр≥в кристал≥зац≥њ, швидк≥стю кристал≥зац≥њ, розм≥ром кристал≥в?
11. як≥ процеси в≥дбуваютьс€ при дозр≥ванн≥ кристал≥чних осад≥в?
12. „ому осадженн€ бар≥й сульфату проводитьс€ з розведених розчин≥в, у кислому середовищ≥, при нагр≥ванн≥ розчин≥в, у присутност≥ солей амон≥ю?
13. як≥ ф≥зико-х≥м≥чн≥ процеси у розчин≥ призвод€ть до сп≥восадженн€?
14. як≥ анал≥тичн≥ заходи застосовуютьс€ дл€ зменшенн€ адсорбц≥њ ≥ оклюз≥њ при отриманн≥ кристал≥чних осад≥в?
15. як≥ умови осадженн€ аморфних осад≥в? „ому њх осадженн€ провод€ть з концентрованих розчин≥в?






