’имический эквивалент
’имическим эквивалентом называетс€ условна€ или реальна€ частица вещества ’, котора€ в данной химической реакции отдаЄт или присоедин€ет элементарный электрический зар€д (без учЄта его знака) или каким-то другим образом соответствует ему.
¬ зависимости от природы носител€ электрического зар€да, которым исходные вещества обмениваютс€ друг с другом, все химические реакции дел€тс€ на 2 типа: реакции ионного обмена (исходные вещества обмениваютс€ ионами) и окислительно-восстановительные реакции (исходные вещества обмениваютс€ электронами).
¬ роли химического эквивалента может выступать молекула (формульна€ единица), атом или ион вещества. ¬ этом случае эквивалент €вл€етс€ реально существующей частицей. Ќо часто он представл€ет собой условную частицу, котора€ в целое число раз меньше молекулы (формульной единицы), атома или иона вещества.
ќдно и то же вещество ’ может иметь несколько химических эквивалентов. ќпределить, что именно представл€ет собой химический эквивалент ’ (реальную частицу или какую-то часть еЄ), можно только, исход€ из конкретной химической реакции, в которой это вещество участвует.
„исло, показывающее, какую часть реальной частицы вещества ’ составл€ет его химический эквивалент, называетс€ иначе фактором эквивалентности и обозначаетс€  fэкв.(’). ‘актор эквивалентности вещества определ€етс€ по формуле
fэкв.(’). ‘актор эквивалентности вещества определ€етс€ по формуле

и €вл€етс€ безразмерной величиной.
ƒл€ вещества, участвующего в реакции ионного обмена, z равно произведению числа ионов, отдаваемых или присоедин€емых одной его молекулой (формульной единицей), на абсолютную величину зар€да одного такого иона.
“аким образом, в реакци€х ионного обмена дл€ кислоты фактор эквивалентности €вл€етс€ величиной, обратной числу ионов Ќ+, замещЄнных в еЄ молекуле на ионы металла или NH4+.
” одноосновных кислот (HCl, HNO3, и т.д.) фактор эквивалентности всегда равен единице. ƒл€ многоосновных кислот (H3PO4, H2S и т.д.) он может принимать несколько значений.
ƒл€ основани€ в реакци€х ионного обмена фактор эквивалентности €вл€етс€ величиной, обратной числу ионов ќЌ-, замещЄнных в его формульной единице на кислотные остатки.
” однокислотных оснований ( ќЌ, NaOH и т.д.) фактор
эквивалентности всегда равен единице. ћногокислотные основани€ (Al(OH)3, Ba(OH)2 и т.д.) могут иметь несколько значений фактора эквивалентности.
ƒл€ соли фактор эквивалентности €вл€етс€ величиной, обратной произведению числа ионов металла (NH4+), содержащихс€ в еЄ формульной единице, на зар€д одного такого иона.
ѕо€сним вышесказанное на примере следующих реакций:
1) H3PO4 + 3KOH = K3PO4 + 3H2O
fэкв.(H3PO4)  =
=  fэкв.(KOH)
fэкв.(KOH) 
’имические эквиваленты исходных веществ будут равны, соответственно:
[fэкв.(H3PO4) H3PO4] = 1/3 H3PO4 (составл€ет 1/3 часть молекулы);
[fэкв.(KOH) KOH] = KOH (совпадает с формульной единицей вещества).
¬ реакции на одну молекулу Ќ3–ќ4 приходитс€ три формульных единицы ќЌ, но на 1Ј3 = 3 химических эквивалента Ќ3–ќ4 приходитс€ такое же количество химических эквивалентов
|
|
|
(3Ј1= 3) ќЌ.
2) H3PO4 + Ca(OH)2 = CaHPO4 + 2H2O
fэкв.(H3PO4)  =
=  fэкв.(Ca(OH)2) =
fэкв.(Ca(OH)2) = 
’имические эквиваленты исходных веществ будут равны, соответственно:
[fэкв.(H3PO4) H3PO4] =  H3PO4 (составл€ет 1/2 часть молекулы);
H3PO4 (составл€ет 1/2 часть молекулы);
[fэкв.(Ca(OH)2)Ca(OH)2] =  Ca(OH)2 (составл€ет 1/2 часть
Ca(OH)2 (составл€ет 1/2 часть
формульной единицы).
¬ реакции на одну молекулу Ќ3–ќ4 приходитс€ одна формульна€ единица Ca(OH)2, но на 1Ј2 =2 химических эквивалента Ќ3–ќ4 приходитс€ такое же количество химических эквивалентов
(1Ј2=2) Ca(OH)2.
3) Al(OH)3 + 2HCl = AlOHCl2 + 2H2O
fэкв.(Al(OH)3) =  fэкв.(HCl)=
fэкв.(HCl)= 
’имические эквиваленты исходных веществ будут равны соответственно:
[fэкв.(Al(OH)3)Al(OH)3] =  Al(OH)3 (составл€ет 1/2 часть
Al(OH)3 (составл€ет 1/2 часть
формульной единицы);
[fэкв.(HCl) HCl] = HCl (совпадает с молекулой вещества).
¬ реакции на одну формульную единицу Al(OH)3 приходитс€ две молекулы HCl, но на 1Ј2=2 химических эквивалента Al(OH)3 приходитс€ такое же количество химических эквивалентов (1Ј 2=2)HCl.
4) Al2(SO4)3 + 3BaCl2 = 3BaSO4↓ + 2AlCl3
fэкв.(Al2(SO4)3) =  fэкв.(BaCl2) =
fэкв.(BaCl2) = 
’имические эквиваленты исходных веществ будут равны, соответственно:
[fэкв.(Al2(SO4)3)Al2(SO4)3] =  Al2(SO4)3 (составл€ет 1/6 часть формульной единицы);
Al2(SO4)3 (составл€ет 1/6 часть формульной единицы);
[fэкв.(BaCl2) BaCl2] =  BaCl2 (составл€ет 1/2 часть формульной единицы).
BaCl2 (составл€ет 1/2 часть формульной единицы).
¬ реакции на одну формульную единицу Al2(SO4)3 приход€тс€ три формульных единицы BaCl2, но на 1Ј6=6 химических эквивалентов Al2(SO4)3 приходитс€ такое же количество химических эквивалентов (3Ј2=6) BaCl2.
ƒл€ вещества ’, участвующего в окислительно-восстановительной реакции (ќ¬–), z €вл€етс€ величиной, равной числу электронов, которые одна его молекула (формульна€ единица) присоедин€ет (если ’ €вл€етс€ окислителем) или отдаЄт (если ’ €вл€етс€ восстановителем) в ходе реакции.
Ќапример, в реакци€х:
1) 3H2S-2 + 2 HN+5 O3 = 3S0 + 2N+2 O + 4 H2O
восстановитель окислитель



S-2 - 2ē = S0 2 3
N+5 + 3ē = N+2 3 2
fэкв.(H2S)=  ; fэкв.(HNO3)=
; fэкв.(HNO3)= 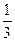 .
.
’имические эквиваленты исходных веществ будут равны, соответственно:
fэкв.(H2S)H2S =  H2S (составл€ет 1/2 часть молекулы),
H2S (составл€ет 1/2 часть молекулы),
fэкв.(HNO3)HNO3= 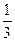 HNO3(составл€ет 1/3 часть молекулы).
HNO3(составл€ет 1/3 часть молекулы).
¬ реакции на три молекулы H2S приходитс€ две молекулы HNO3, но на 3Ј2=6 химических эквивалентов H2S приходитс€ такое же количество химических эквивалентов (2Ј3=6) HNO3.
2)5H2C2+3O4+2KMn+7O4+3Ќ2Sќ4=10C+4O2+K2SO4+ 2Mn+2SO4 +8H2O
восстановитель окислитель
 |  | ||||
 | |||||
2—+3 - 2ē = 2—+4 2 5
Mn+7 + 5ē = Mn+2 5 2
fэкв.(H2C2O4)=  ; fэкв.(KMnO4) =
; fэкв.(KMnO4) =  .
.
’имические эквиваленты исходных веществ будут равны, соответственно:
fэкв.(H2C2O4)H2C2O4=  H2C2O4 (составл€ет 1/2 часть молекулы);
H2C2O4 (составл€ет 1/2 часть молекулы);
fэкв.(KMnO4)KMnO4 =  KMnO4 (составл€ет 1/5 часть формульной единицы).
KMnO4 (составл€ет 1/5 часть формульной единицы).
¬ реакции на п€ть молекул H2C2O4 приходитс€ две формульных единицы KMnO4, но на 5Ј2=10 химических эквивалентов H2C2O4 приходитс€ такое же количество химических эквивалентов (2Ј5=10) KMnO4.
3) 2—0 + 2HN+5 O3 = 2C+4O2 + N2+1O + H2O
восстановитель окислитель


 —0 - 4ē = —+4 4 2
—0 - 4ē = —+4 4 2
2N+5 + 8ē = 2N+1 8 1
fэкв.(—)=  ; fэкв.(HNO3)=
; fэкв.(HNO3)=  .
.
’имические эквиваленты исходных веществ будут равны, соответственно:
|
|
|
fэкв.(—)—=  — (составл€ет ¼ часть атома);
— (составл€ет ¼ часть атома);
fэкв.(HNO3)HNO3=  HNO3 (составл€ет 1/4 часть молекулы).
HNO3 (составл€ет 1/4 часть молекулы).
¬ реакции на два атома — приходитс€ две молекулы HNO3 и на 2Ј1=2 химических эквивалента — приходитс€ такое же количество химических эквивалентов (2Ј1=2) HNO3.
оличества прореагировавших молекул (формульных единиц) исходных веществ в большинстве химических реакций не относ€тс€ друг к другу как 1:1. »сключение составл€ют те случаи, когда в уравнении реакции перед формулами реагентов сто€т одинаковые стехиометрические коэффициенты. Ќо в любой химической реакции количества расходованных химических эквивалентов исходных веществ равны между собой.






