ѕример 10. ¬ычислить значение рЌ раствора, полученного при сливании 20,0 мл 0,1 ћ раствора NaOH и 16,0 мл 0,08 ћ раствора HCl.
–ешение. «апишем уравнение реакции, котора€ протекает после сливани€ двух растворов:
NaOH + HCl = NaCl + H2O
— целью вы€снени€ состава раствора, образовавшегос€ после сливани€, рассчитаем количества веществ в исходных растворах:
n 0(NaOH) = 20,0 ∙ 10Ц3 ∙ 0,1 = 2,0 ∙ 10Ц3 моль;
n 0(HCl) = 16,0 ∙ 10Ц3 ∙ 0,08 = 1,28 ∙ 10Ц3 моль.
“ак как n 0(NaOH) > n 0(HCl), то NaOH находитс€ в избытке, следовательно, в образовавшемс€ после сливани€ растворе будут находитьс€ NaOH и NaCl в следующих количествах:
n 1(NaOH) = n 0(NaOH) Ц n 0(HCl) = 2,0 ∙ 10Ц3 Ц 1,28 ∙ 10Ц3 =
= 0,72 ∙ 10Ц3 моль.
n 1(NaCl) = n 0(HCl) = 1,28 ∙ 10Ц3 моль.
NaCl €вл€етс€ солью сильной кислоты и сильного основани€ и на pH не вли€ет.
ќбъем раствора после сливани€ (V) составит 20,0 + 16,0 = 36,0 мл.
–ассчитаем концентрацию NaOH в растворе:
— (NaOH) = 0,72 ∙ 10Ц3/ 36 ∙ 10Ц3 = 0,02 моль/л.
»сход€ из состава раствора, выбираем формулу (3.8) дл€ расчета рЌ в растворе сильного основани€:
pH = 14 + lg C (NaOH) = 14 + lg(0,02) = 12,3
ѕример 11. ¬ычислить значение рЌ раствора, полученного при сливании 20,0 мл 0,1 ћ раствора NaOH и 36,0 мл 0,08 ћ раствора HCl.
–ешение. «апишем уравнение реакции, котора€ протекает после сливани€ двух растворов:
NaOH + HCl = NaCl + H2O
— целью вы€снени€ состава раствора, образовавшегос€ после сливани€, рассчитаем количества веществ в исходных растворах:
n 0(NaOH) = 20,0 ∙ 10Ц3 ∙ 0,1 = 2,0 ∙ 10Ц3 моль;
n 0(HCl) = 36,0 ∙ 10Ц3 ∙ 0,08 = 2,88 ∙ 10Ц3 моль.
“ак как n 0(NaOH) < n 0(HCl), то HCl находитс€ в избытке, следовательно, в образовавшемс€ после сливани€ растворе будут присутствовать HCl и NaCl в следующих количествах:
n 1(HCl) = n 0(HCl) Ц n 0(NaOH) = 2,88 ∙ 10Ц3 Ц 2,0 ∙ 10Ц3 =
= 0,88 ∙ 10Ц3 моль
n 1(NaCl) = n 0(NaOH) = 2,0 ∙ 10Ц3 моль
NaCl €вл€етс€ солью сильной кислоты и сильного основани€ и на pH не вли€ет.
ќбъем раствора (V) составит 20,0 + 36,0 = 56,0 мл.
–ассчитаем концентрацию HCl в растворе:
— (HCl) = 0,88 ∙ 10Ц3/ 56 ∙ 10Ц3 = 0,0157 моль/л.
»сход€ из состава раствора, выбираем формулу (3.3) дл€ расчета рЌ в растворе сильной кислоты:
pH = lg C (HCl) = lg(0,0157) = 1,8.
ѕример 12. ¬ычислить значение рЌ раствора, полученного при сливании 20,0 мл 0,12 ћ раствора NaCN и 15,0 мл 0,09 ћ раствора HCl.
–ешение. «апишем уравнение реакции, котора€ протекает после сливани€ двух растворов:
NaCN + HCl = HCN + NaCl
— целью вы€снени€ состава раствора, образовавшегос€ после сливани€, рассчитаем количества веществ в исходных растворах:
n 0(NaCN) = 20,0 ∙ 10Ц3 ∙ 0,12 = 2,4 ∙ 10Ц3 моль;
n 0(HCl) = 15,0 ∙ 10Ц3 ∙ 0,09 = 1,35 ∙ 10Ц3 моль.
“ак как n 0(NaCN) > n 0(HCl), то NaCN находитс€ в избытке, следовательно, в образовавшемс€ после сливани€ растворе будут присутствовать NaCN и HCN в следующих количествах:
|
|
|
n 1(NaCN) = n 0(NaCN) Ц n 0(HCl) = 2,4 ∙ 10Ц3 Ц 1,35 ∙ 10Ц3 =
= 1,05 ∙ 10Ц3 моль;
n 1(HCN) = n 0(HCl) = 1,35 ∙ 10Ц3 моль.
ќбъем раствора после сливани€ (V) составит 20 + 15 = 35 мл.
–ассчитаем концентрации веществ в растворе:
— (NaCN) = 1,05 ∙ 10Ц3/ 35 ∙ 10Ц3 = 0,03 моль/л;
— (HCN) = 1,35 ∙ 10Ц3/ 35 ∙ 10Ц3 = 0,039 моль/л.
»сход€ из состава раствора, выбираем формулу (3.15) дл€ расчета рЌ буферных растворов:
pH =  Ц lg[ — (HCN) / — (NaCN)] = 9,3 Ц lg(0,039 / 0,03) = 9,18
Ц lg[ — (HCN) / — (NaCN)] = 9,3 Ц lg(0,039 / 0,03) = 9,18
ѕример 13. ¬ычислить значение рЌ раствора, полученного при сливании 11,25 мл 0,12 моль/л раствора NaCN и 15,0 мл 0,09 моль/л раствора HCl.
–ешение. «апишем уравнение реакции, котора€ протекает после сливани€ двух растворов, и рассчитаем количества веществ:
NaCN + HCl = HCN + NaCl
n (NaCN) = 11,25 ∙ 10Ц3 ∙ 0,12 = 1,35 ∙ 10Ц3 моль;
n (HCl) = 15,0 ∙ 10Ц3 ∙ 0,09 = 1,35 ∙ 10Ц3 моль.
“ак как n (NaCN) = n (HCl), то в образовавшемс€ после сливани€ растворе будет находитьс€ только 1,35 ∙ 10Ц3 моль HCN.
ќбъем раствора (V) составит 11,25 + 15,0 = 26,25 мл.
–ассчитаем концентрацию HCN в растворе:
— (HCN) = 1,35 ∙ 10Ц3/ 26,25 ∙ 10Ц3 = 0,051 моль/л.
»сход€ из состава раствора, выбираем формулу (3.5) дл€ расчета рЌ в растворе слабой кислоты:
pH = 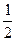
 Ц
Ц 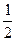 lg[ — (HCN)] =
lg[ — (HCN)] = 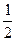 ∙ 9,3 Ц
∙ 9,3 Ц 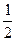 ∙ lg(0,051) = 5,3
∙ lg(0,051) = 5,3
ѕример 14. ¬ычислить значение рЌ раствора, полученного при сливании 20,0 мл 0,12 моль/л раствора NaCN и 35,0 мл 0,09 моль/л раствора HCl.
–ешение. «апишем уравнение реакции, котора€ протекает после сливани€ двух растворов, и рассчитаем количества веществ:
NaCN + HCl = HCN + NaCl
n 0(NaCN) = 20,0 ∙ 10Ц3∙ 0,12 = 2,4 ∙ 10Ц3 моль;
n 0(HCl) = 35,0 ∙ 10Ц3 ∙ 0,09 = 3,15 ∙ 10Ц3 моль.
“ак как n 0(HCl) > n 0(NaCN), то в образовавшемс€ после сливани€ растворе будут находитьс€ HCl и HCN в следующих количествах:
n 1(HCl) = 3,15 ∙ 10Ц3 Ц 2,4 ∙ 10Ц3 = 0,75 ∙ 10Ц3 моль;
n 1(HCN) = 20,0 ∙ 10Ц3 ∙ 0,12 = 2,4 ∙ 10Ц3 моль.
ѕоскольку сильна€ кислота HCl подавл€ет диссоциацию слабой кислоты HCN, то рЌ определ€етс€ только ее концентрацией.
ќбъем раствора (V) составит 20,0 + 35,0 = 55,0 мл.
–ассчитаем концентрацию HCl в растворе:
— (HCl) = 0,75 ∙ 10Ц3/ 55,0 ∙ 10Ц3 = 0,014 моль/л.
»сход€ из состава раствора, выбираем формулу (3.3) дл€ расчета рЌ в растворе сильной кислоты:
pH = Ц lg[—(HCl)] = Ц lg(0,014) = 1,87.
ѕример 15. ¬ычислить значение рЌ раствора, полученного при сливании 10,0 мл 0,1 моль/л раствора Na2HAsO4 и 16,0 мл 0,1 моль/л раствора HCl.
–ешение. ѕосле сливани€ растворов могут протекать следующие реакции:
Na2HAsO4 + HCl = NaH2AsO4 + NaCl (3.16)
NaH2AsO4 + HCl = H3AsO4 + NaCl (3.17)
–ассчитаем количества вещества в исходных растворах:
n (Na2HAsO4) = 10,0 ∙ 10Ц3 ∙ 0,1 = 1,0 ∙ 10Ц3 моль;
n (HCl) = 16,0 ∙ 10Ц3 ∙ 0,1 = 1,6 ∙ 10Ц3 моль.
“ак как Na2HAsO4 вз€т в недостатке, то все количество его прореагирует с HCl согласно уравнению (3.16), и после протекани€ реакции (3.16) в растворе останетс€ 1,0 ∙ 10Ц3 моль NaH2AsO4 и
(1,6 Ц 1,0) ∙ 10Ц3 = 0,6 ∙ 10Ц3 моль HCl. јналогично после завершени€ реакции (3.17) в растворе будут находитьс€ H3AsO4 и NaH2AsO4 в следующих количествах:
|
|
|
n (NaH2AsO4) = 1,0 ∙ 10Ц3 Ц 0,6 ∙ 10Ц3 = 0,4 ∙ 10Ц3 моль;
n (H3AsO4) = 0,6 ∙ 10Ц3 моль.
ќбъем раствора (V) после смешени€ составит 10 + 16 = 26 мл, или 26 ∙ 10Ц3 л.
–ассчитаем концентрации компонентов в растворе:
— (NaH2AsO4) = 0,4 ∙ 10Ц3/ 26 ∙ 10Ц3 = 0,015 моль/л;
— (H3AsO4) = 0,6 ∙ 10Ц3/ 26 ∙ 10Ц3 = 0,023 моль/л.
»сход€ из состава раствора, выбираем формулу (3.15) дл€ расчета рЌ буферных растворов:
pH = 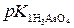 Ц lg(— (H3AsO4) / — (NaH2AsO4)) =
Ц lg(— (H3AsO4) / — (NaH2AsO4)) =
= 2,22 Ц lg(0,023/0,015) = 2,03.






