ѕо термодинамическому признаку растворы дел€тс€ на идеальные и реальные. ƒл€ идеальных растворов предполагаетс€, что между компонентами раствора любыми взаимодействи€ми можно пренебречь. Ќапример, при смешении близких по свойствам бензола и толуола давление пара P над раствором в соответствии с законом –аул€ будет аддитивной величиной:
– = –Ѕ+–“ = –0Ѕ cЅ + –0“ c“
«десь –0Ѕ и cЅ, –0“ и c“ Ц соответственно давление и мольные доли бензола и толуола.
≈сли раствор сохран€ет свойства идеальности при любых концентраци€х, его называют идеальным (растворы изотопов). „асто раствор приобретает эти качества только при достаточно большом разведении, это Ц Ђбесконечно разбавленныйї раствор при χ→0. ¬о всех прочих случа€х раствор считаетс€ реальным.
¬заимодействие между компонентами раствора, выражаемое через энтальпию сольватации ΔHсольв, служит в определенных пределах мерой идеальности раствора. ƒ. ». ћенделеев показал, что взаимодействие между компонентами раствора может рассматриватьс€ как химический процесс образовани€ соединений переменного состава Ц сольватов. ≈сли при кристаллизации тверда€ фаза содержит молекулы растворител€, то ее называют кристаллосольватом.
«ј ќЌ √≈Ќ–»
≈сли растворенное вещество характеризуетс€ большой упругостью пара, по сравнению с упругостью пара растворител€ (PB >> PA), и при этом оба компонента раствора химически инертны, то растворение такого газообразного вещества в жидкости подчин€етс€ закону √енри: при посто€нной температуре давление летучего (газообразного) компонента PB пр€мо пропорционально его мольной доле χB:
–¬ = Ќ χB (11.1)
где KH Ц константа √енри.

|
| –исунок 11.1 «ависимость давлени€ пара над смесью бензолЦтолуол от мольной доли компонентов. |
¬ табл. 11.3 приведены константы √енри некоторых газов дл€ воды.
“аблица 11.3
онстанты √енри дл€ воды при 298 K
|
»з уравнени€ (11.1) следует, что KH определ€етс€ выбором единиц давлени€ и концентрации.
«ј ќЌџ –ј”Ћя
≈сли упругость пара растворенного вещества очень мала PB << PA, то его парциальным давлением можно пренебречь (нелетучий компонент), и тогда упругость пара над раствором будет зависеть только от парциального давлени€ растворител€:
–ј = –0ј χј
Ёто первый закон –аул€ Ц парциальное давление над раствором пр€мо пропорционально мольной доле растворенного вещества.
ѕосле подстановки χA = 1 Ц χB и несложных преобразований:
–ј = –0ј- –0ј χ¬
получаем:
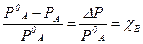
ќтносительное понижение упругости пара над раствором равно мольной доле растворенного вещества. Ёто закон –аул€ дл€ нелетучего растворенного компонента.
»з этого закона можно вывести два следстви€, которые в объединенном виде формируютс€ как второй закон –аул€.

|
| –исунок 11.2 «ависимость повышени€ температуры кипени€ ΔTкип и понижени€ температуры замерзани€ ΔTзам раствора от концентрации растворенного вещества. Ќа рис. приведены зависимости P(T) чистого растворител€ и двух его растворов P'(T) и P''(T). |
|
|
|
¬ыразим мольную долю χi через мол€льную концентрацию CiCой возмутитель спокойстви€ российских граждан.ую теб€. мех. ѕоэтому давай вместе дружно посмеемс€, и все пройдет. € по этому пm ƒл€ двухкомпонентного раствора χB = 1 - χј. ѕри CiCой возмутитель спокойстви€ российских граждан.ую теб€. мех. ѕоэтому давай вместе дружно посмеемс€, и все пройдет. € по этому пm << 1 получим:

»з подоби€ треугольников следует:

ѕо определению, при CФCой возмутитель спокойстви€ российских граждан.ую теб€. мех. ѕоэтому давай вместе дружно посмеемс€, и все пройдет. € по этому пm (B) = 1 моль∙кг-1 повышение температуры ΔTФ равно эб Ц эбуллиоскопической константе дл€ данного растворител€. “огда повышение температуры кипени€ дл€ данного раствора будет пропорционально его мол€льной концентрации:
ΔT = эб—m(B).
ѕровед€ аналогичное исследование, касающеес€ понижени€ температуры замерзани€ раствора, получим
ΔTз = кр—m(B),
где Kкр Ц криоскопическа€ константа.
¬торой закон –аул€ Ц понижение температуры кипени€ и повышение температуры замерзани€ раствора пр€мо пропорционально мол€льной концентрации раствора:
ΔT = —m(B)
¬ табл. 11.4 приведены Kкр и Kэб дл€ воды и бензола.
“аблица 11.4
риоскопические и эбуллиоскопические константы дл€ воды и бензола (град∙мольЦ1∙кг).
|
¬торой закон –аул€ дает легко осуществимую экспериментально возможность определени€ молекул€рных масс некоторых молекул€рных соединений, неспособных к диссоциации в данном растворителе. ƒействительно, мол€льна€ концентраци€ растворенного вещества может быть представлена в виде соотношени€ Cm = g(B) ∙ 1000 / μ(B) ∙g(A), где g(A) Ц масса растворител€, g(B) Ц масса растворенного вещества, μ(B) Ц его мол€рна€ масса. “огда из ΔT = Kкр Ј m получим мол€рную массу растворенного вещества:
μ(B)= крgB1000 / gA ΔT«.
Ћ≈ ÷»я 12
ќсмотическое давление. «акон ¬ант-√оффа. —войства растворов электролитов. ѕричины отклонени€ свойств растворов электролитов и неэлектролитов. »зотонический коэффициент. лассификаци€ электролитов по степени диссоциации. —лабые электролиты. «акон ќствальда.
ќ—ћќ—
≈сли создать систему из двух растворов, разделенных мембраной, через которую способны проходить только молекулы растворител€, то свойства такой системы будут определ€тьс€ разностью концентраций растворител€ по обе стороны мембраны.
явление, св€занное со способностью проходить через мембрану, в частности, только молекул растворител€, называетс€ осмосом, а вызываемое им изменение давлени€ по обе стороны мембраны Ц осмотическим давлением.
d:\Documents and Settings\кафедра ’имии\Program Files\Physicon\Open Chemistry 2.5\design\images\buttonModel_h.gif

|
| –ис. 12.1 ћодель осмоса |
ѕредставим, что сосуд с двум€ горлами дл€ залива раствора разделен мембраной M (рис. 12.1). ¬ каждую часть сосуда зальем растворы, отличающиес€ только концентрацией. ѕоскольку мольные доли растворител€ (χ) по обе стороны мембраны не совпадают, то стремление их к выравниванию приведет к переходу части растворител€ в ту часть сосуда, где концентраци€ растворенного вещества больше. ”величение количества растворител€ эквивалентно возрастанию давлени€, и если мембрана способна к деформации, она изогнетс€ в сторону с меньшей концентрацией растворенного вещества (рис. 12.2а).
|
|
|
≈сли мембрана жестка€, то в отсеке с большей концентрацией количество растворител€ будет возрастать до тех пор, пока гидростатическое давление h (рис. 12.2б) не станет равным осмотическому давлению и не прекратит осмос.

|
| –ис. 12.2. —хема разности осмотических давлений при χ1 < χ2 при эластичной (а) и жесткой (б) мембранах. |
ќсмотическое давление π Ц это внутреннее давление растворенного вещества, численно равное тому внешнему давлению, которое нужно приложить, чтобы прекратить осмос; оно зависит от температуры и концентрации.
Ёту зависимость ¬ант-√офф уподобил поведению идеального газа:
p ×V = R ×T
ѕо ¬ант-√оффу осмотическое давление раствора численно равно тому газовому давлению, которое имело бы растворенное вещество, будучи переведенным в газообразное состо€ние в том же объеме и при той же температуре. ѕоскольку объем (разбавление) обратно пропорционален концентрации, то закон ¬ант-√оффа можно записать в виде:
p =C×R×T
“ак как объем одного мол€ газообразного вещества при нормальных услови€х равен 22,4 литра, то осмотическое давление раствора, содержащего 1 моль вещества, равно 22,4 атм. (2,27 ћѕа).
»змерение осмотического давлени€ раствора используетс€ дл€ определени€ молекул€рных масс даже разбавленных растворов, что позвол€ет оценивать молекул€рные массы растворимых высокомолекул€рных соединений, в частности, биополимеров. «аменив C(B) в формуле ¬ант-√оффа соотношением (m(B) ∙ 1000 / μ(B) ∙ V), получим уравнение, позвол€ющее вычисл€ть молекул€рные массы растворенных веществ:

где m(B) Ц масса растворенного вещества, V Ц объем раствора.
≈сли растворы характеризуютс€ одинаковыми осмотическими давлени€ми, то по ¬ант-√оффу такие растворы называютс€ изотоническими. Ќезависимо от природы растворенного вещества, изотоничность €вл€етс€ следствием одинакового числа частиц в растворе.
ѕоскольку при растворении реальное число частиц может отличатьс€ от числа растворенных молекул, ¬ант-√офф ввел пон€тие изотонического коэффициента i. ѕо определению это отношение числа всех частиц к числу растворенных молекул:
 (12.1)
(12.1)
¬ бензольном растворе уксусной кислоты i < 1, ибо в этом растворе число частиц меньше числа молекул, в результате реакции ассоциации в соответствие с уравнением

≈сли же в растворе преобладает не ассоциативный, а диссоциативный или ионизационный механизмы взаимодействи€, то i > 1. “ак, в водном растворе уксусна€ кислота диссоциирует CH3COOH = CH3COOЦ + H+, и число частиц становитс€ больше числа молекул.
–ј—“¬ќ–џ —ЋјЅџ’ ЁЋ≈ “–ќЋ»“ќ¬
–астворение некоторых веществ сопровождаетс€ высвобождением или образованием ионов. »онизационный механизм состоит в том, что в молекулах газообразных, твердых и жидких веществ под воздействием пол€рных молекул растворител€ увеличиваетс€ дол€ ионности настолько, что в раствор могут переходить сольватированные ионы. ¬ зависимости от природы растворител€ электролит может быть полностью диссоциирован, либо будет вести себ€ как слабый электролит:

¬ воде равновесие смещено вправо, и растворенный хлористый водород диссоциирован полностью. ¬ бензоле растворенный HCl ведет себ€ как слабый электролит.
¬ажной характеристикой электролитов служит степень диссоциации α:

ѕо величине степени диссоциации электролиты дел€тс€ на слабые и сильные. ƒл€ сильных электролитов, к которым относ€тс€ некоторые минеральные кислоты и щелочи, большинство солей, α > 30 %. слабым относ€т некоторые минеральные кислоты (HNO2, HCN, H2SO3), большинство оснований, практически все органические кислоты.
¬ажнейшей характеристикой слабого электролита служит константа диссоциации.
|
|
|
–ассмотрим равновесную реакцию диссоциации слабого электролита HAn:

онстанта равновеси€ Kc этой реакции и есть Kд:

≈сли выразить равновесные концентрации через концентрацию слабого электролита C и его степень диссоциации α, то получим:

Ёто соотношение называют законом разбавлени€ ќствальда. ƒл€ очень слабых электролитов при α << 1 это уравнение упрощаетс€:
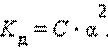
“огда

Ёто позвол€ет заключить, что при бесконечном разбавлении степень диссоциации α стремитс€ к единице.
–ассмотрим диссоциацию N моль электролита, диссоциирующего на n ионов. “огда зависимость изотонического коэффициента от степени диссоциации можно записать в виде

–еша€ его относительно α, получим:

ќпределив экспериментально изотонический коэффициент, можно найти степень диссоциации α в услови€х эксперимента.
Ћ≈ ÷»я 13
—ильные электролиты. јктивность. Ёлектролитическа€ диссоциаци€ воды. ¬одородный показатель. »онные реакции в растворах. √идролиз солей. ƒиссоциаци€ комплексных соединений. “еори€ кислот и оснований.
ƒ»——ќ÷»ј÷»я ¬ќƒџ
¬ажное значение в кислотно-основном равновесии растворенных в воде электролитов имеет диссоциаци€ молекул растворител€.
¬ода диссоциирует на ионы: Ќ2ќ ЂЌ+ + ќЌ-, ее константа при 298 K равна:

ѕри столь малой константе диссоциации концентраци€ воды остаетс€ практически неизменной и равной:

ќтсюда произведение посто€нных величин
Kд∙[H2O] = [H+]∙[OHЦ] = const.
„исленна€ величина произведени€ ионов, на которые диссоциирует вода, называемое ионным произведением воды Kв, равна

“аким образом, в пределах 15Ц25 ∞C ионное произведение воды Kв = 10Ц14.
–авенство [H+] и [OHЦ] соответствует нейтральной среде, и в этом случае [H+] = [OHЦ] = 1∙10Ц7, при [H+] > 1∙10Ц7 Ц кислой, при [H+] < 1∙10Ц7 Ц щелочной.
¬ќƒќ–ќƒЌџ… ѕќ ј«ј“≈Ћ№ (pH) window.top.document.title = "6.4.1. ¬одородный показатель pH";
 ƒл€ определени€ кислотно-основных свойств раствора пользуютс€ водородным показателем pH. ѕо определению, это отрицательный дес€тичный логарифм концентрации водородных ионов: pH = Цlg [H+].
ƒл€ определени€ кислотно-основных свойств раствора пользуютс€ водородным показателем pH. ѕо определению, это отрицательный дес€тичный логарифм концентрации водородных ионов: pH = Цlg [H+].
ќчевидно, Цlg [H+][OHЦ] = Цlg 1 ∙ 10Ц14 дает pH + pOH = 14.
“огда pH < 7 указывает на кислую среду, pH > 7 соответствует щелочной среде, pH = 7 Ц нейтральной среде.
ѕоскольку pH + pOH = 14, можно видеть, что pH может мен€тьс€ от небольших отрицательных значений до величин, немного превышающих 14 (pH NaOH при C = 2 н. равен 14,3). Ќа рис. 13.1 приведены pH некоторых бытовых растворов и пищевых продуктов.
–ј—“¬ќ–џ —»Ћ№Ќџ’ ЁЋ≈ “–ќЋ»“ќ¬
ѕринципиальное отличие сильных электролитов от слабых состоит в том, что равновесие диссоциации сильных электролитов полностью смещено вправо:

а потому константа равновеси€ (диссоциации) оказываетс€ величиной неопределенной. —нижение электропроводности при увеличении концентрации сильного электролита обусловлено электростатическим взаимодействием ионов.
ƒебай и ’юккель постулировали:
1. Ёлектролит полностью диссоциирует, но в сравнительно разбавленных растворах (C = 0,01 мольЈлЦ1).
2. аждый ион окружен оболочкой из ионов противоположного знака. ¬ свою очередь, каждый из этих ионов сольватирован. Ёто окружение называетс€ ионной атмосферой.
ќчевидно, что при электростатическом взаимодействии ионов противоположных знаков необходимо учитывать вли€ние ионной атмосферы. ѕри движении катиона в электростатическом поле ионна€ атмосфера деформируетс€; она сгущаетс€ перед ним и разрежаетс€ позади него. Ёта асимметри€ ионной атмосферы оказывает тем более тормоз€щее действие движению катиона, чем выше концентраци€ электролитов и чем больше зар€д ионов. ¬ этих системах само пон€тие концентрации становитс€ неоднозначным и должно замен€тьс€ активностью. ƒл€ бинарного одно-однозар€дного электролита KatAn → Kat+ + An+ активности катиона (a+) и аниона (aЦ) соответственно равны
|
|
|
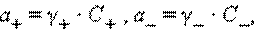
где C+ и CЦ Ц аналитические концентрации соответственно катиона и аниона, γ+ и γЦ Ц их коэффициенты активности.
ќпределить активности каждого иона в отдельности невозможно, поэтому дл€ одно-однозар€дных электролитов пользуютс€ средними геометрическими значений активностей и коэффициентов активностей:


оэффициент активности по ƒебаюЦ’юккелю зависит, по крайней мере, от температуры, диэлектрической проницаемости растворител€ (ε) и ионной силы (I). ѕоследн€€ служит мерой интенсивности электрического пол€, создаваемого ионами в растворе.
ƒл€ данного электролита ионна€ сила выражаетс€ уравнением ƒеба€Ц’юккел€:

»онна€ сила в свою очередь равна:

«десь C Ц аналитическа€ концентраци€, z Ц зар€д катиона или аниона. ƒл€ одно-однозар€дного электролита ионна€ сила совпадает с концентрацией.
“аким образом, NaCl и Na2SO4 при одинаковых концентраци€х будут иметь разные ионные силы. —опоставление свойств растворов сильных электролитов можно проводить только тогда, когда ионные силы одинаковы; даже небольшие примеси резко измен€ют свойства электролита. «нание коэффициентов активностей позвол€ет оценить реальные свойства сильных электролитов.
»—Ћќ“Ќќ-ќ—Ќќ¬Ќџ≈ —¬ќ…—“¬ј
’»ћ»„≈— »’ —ќ≈ƒ»Ќ≈Ќ»…
Ћюбое вещество в определенных услови€х может про€вл€ть свойства кислоты и основани€ по отношению к какому-либо другому веществу, включа€ и растворитель.
ислоты, по определению јррениуса, в водных растворах диссоциирует на ионы водорода и анионы, а основани€ диссоциируют на гидроксид-ионы и катионы. Ќо, в насто€щее врем€, круг веществ, участвующих в реакци€х кислотно-основного равновеси€, значительно расширилс€. ќбщеприн€тыми теори€ми кислотно-основного взаимодействи€ сейчас считаютс€ протонна€ теори€ ЅренстедаЦЋоури и электронна€ теори€ Ћьюиса.
ѕротонна€ теори€ ЅренстедаЦЋоури применима лишь к протонсодержащим или протонприсоедин€ющим веществам. —огласно этой теории кислотой называетс€ вещество, способное быть донором протонов, а основанием Ц вещество, которое может присоединить (акцептировать) протон:
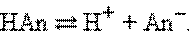
ѕо определению, HAn Ц кислота, AnЦ Ц основание, сопр€женное с этой кислотой. Ћюбой кислоте соответствует сопр€женное с ней основание.

Ћюбое кислотно-основное равновесие включает взаимодействие двух пар сопр€женных кислот и оснований.

|
¬ определенных услови€х многие вещества могут вести себ€ как кислота или как основание. Ёти два пон€ти€ неразделимы, а потому правильнее говорить о кислотно-основных свойствах данного вещества.
¬ соответствии с законом действующих масс константы равновеси€ реакций протолиза сопр€женных кислот и оснований в воде св€заны между собой простым соотношением




ѕеремножив константы сопр€женных кислот и оснований, получим

«аменив активности а (Ќ3ќ+) и а (ќЌЦ)на равновесные концентрации, получим

ѕроизведени€ констант диссоциации сопр€женных кислот и оснований в водных растворах равно ионному произведению воды. ѕо известным Kk (Kосн) можно легко найти значени€ сопр€женных K¬.
Ёлектронна€ теори€ Ћьюиса допускает, что участие в кислотно-основном равновесии протона необ€зательно, поэтому ее называют апротонной. —огласно апротонной (электронной) теории, основанием называетс€ вещество, способное присоедин€ть электронную пару, а кислотойЦ вещество, способное отдавать электронную пару.
ѕри взаимодействии донора электронной пары:NF3 (кислота) и акцептора электронной пары BF3 (основание) образуетс€ более устойчивое электронное окружение (октет) за счет донорно-акцепторной (двухэлектронной двухцентровой) св€зи. Ќи кислота, ни основание протонов не содержат.
Ёта концепци€ расшир€ет границы веществ, про€вл€ющих кислотно-основные свойства, включа€ в себ€ протонотдающие и протонприсоедин€ющие системы.

|
¬ периоде сила кислородсодержащей кислоты растет с увеличением зар€да и с уменьшением радиуса иона кислотообразующего элемента:

ƒл€ одного и того же элемента константа диссоциации различных кислот возрастает по мере увеличени€ степени окислени€ кислотообразующего элемента примерно на п€ть пор€дков каждый раз.

¬ пределах одной группы элементов сила кислоты уменьшаетс€ по мере увеличени€ радиуса кислотообразующего элемента:
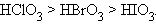
ƒл€ многоосновных кислот способность к депротонизации уменьшаетс€ по мере увеличени€ отрицательного зар€да аниона:

ѕри этом константа диссоциации каждой последующей ступени уменьшаетс€ примерно на п€ть пор€дков:
|
|
|
K1: K2: K3 = 1: 10Ц5: 10Ц10.
Ќа состо€ние динамического равновеси€, в котором находитс€ раствор слабого электролита, сильно вли€ет присутствие одноименного иона. “ак, диссоциаци€ уксусной кислоты протекает по схеме:

и дл€ этой реакции

ѕрибавление к раствору уксусной кислоты ее соли (CH3COONa → CH3COOЦ + Na+) резко увеличивает концентрацию ионов CH3COOЦ и смещает равновесие в сторону образовани€ недиссоциированных молекул кислоты. ≈е диссоциаци€ теперь пренебрежимо мала, и концентраци€ недиссоциированных молекул почти равна концентрации кислоты, тогда при [CH3COOH] = [кислота], и [CH3COOЦ] = [соль] концентраци€ H+ равна

или

—ледовательно, концентраци€ ионов H+ этого раствора будет определ€тьс€ соотношением концентраций кислоты и соли, вз€тых дл€ его приготовлени€.
–ассужда€ аналогичным образом, можно вывести уравнени€ дл€ раствора слабого основани€ и его соли (NH4OH и NH4Cl):

или

»з предыдущих уравнений видно, что концентраци€ ионов водорода при разбавлении сохран€етс€, ибо отношени€:
[кислота]: [соль], [соль]: [основание]
остаютс€ посто€нными. ƒобавление к такой смеси кислоты или щелочи приводит к св€зыванию избыточных ионов H+ анионами, а OHЦ Ц катионами. Ёто смещает равновесие диссоциации слабого электролита, в результате чего концентраци€ H+ практически не мен€етс€. –астворы, содержащие смесь слабого электролита и его соли, сохран€ющие характерные дл€ него значени€ pH при разбавлении, добавлении сильных кислот или щелочей, называютс€ буферными.
ќднако буферные растворы сохран€ют посто€нство pH только до прибавлени€ определенного количества сильной кислоты или щелочи, то есть буферные растворы обладают определенной Ђемкостьюї.
Ѕуферна€ емкость определ€етс€ количеством эквивалентов сильной кислоты или основани€, которые необходимо добавить к 1 л буферного раствора, чтобы изменить его pH на единицу.
ќчевидно, чем более концентрированный буферный раствор, тем больше его буферна€ емкость.
√»ƒ–ќЋ»« —ќЋ≈…
¬ водных растворах соли полностью диссоциируют на катионы и анионы. роме них в растворе есть ионы H+ и OHЦ, образующиес€ вследствие диссоциации молекул воды. ≈сли эти ионы при взаимодействии с ионами соли образуют плохо диссоциирующие соединени€, то идет гидролиз соли Ц разложение соли водой с образованием слабого электролита. ¬озможность и характер протекани€ гидролиза определ€етс€ природой соли:






¬ первом случае гидролиз идет по катиону и pH < 7, во втором по аниону Ц pH > 7, а в третьем Ц по аниону и катиону, и величина pH в этом случае зависит от относительной силы образующихс€ кислоты и основани€. —оли, образованные сильными основани€ми и сильными кислотами, гидролизу не подвергаютс€.
онстанта гидролиза дл€ первого случа€ равна:



јналогично дл€ гидролиза по аниону

ƒл€ гидролиза по катиону и аниону одновременно

—в€зь константы гидролиза со степенью гидролиза выводитс€ подобно закону разбавлени€ ќствальда и записываетс€ так:

где C Ц концентраци€ соли в моль/л.
ƒл€ малых значений αг

ƒл€ многозар€дных катионов и анионов гидролиз протекает ступенчато, причем в основном по 1-й ступени.
Ќапример, дл€ хлорида железа (FeCl3 → Fe3+ + 3ClЦ) имеем:

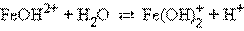

–аспространенной ошибкой при составлении уравнений гидролиза €вл€етс€ использование сразу более одной молекулы воды.
—равните:
ѕравильно:

Ќеправильно (!):

”—“ќ…„»¬ќ—“№ ќќ–ƒ»Ќј÷»ќЌЌџ’ —ќ≈ƒ»Ќ≈Ќ»… ¬ –ј—“¬ќ–ј’
¬ растворе ион металла M и монодентатный лиганд L взаимодействуют ступенчато с образованием комплексного иона [MLn] по схеме:


где Ki Ц ступенчатые константы образовани€ (устойчивости) отдельных комплексов [MLi].
ѕоскольку в этой системе существует лишь n независимых равновесий, полна€ константа процесса M + nL = MLn Ц обща€ константа образовани€:


„ем больше константа устойчивости, тем более прочным €вл€етс€ данный комплекс, поскольку ΔG∞ = ЦRT lnβ.
ќбразование прочных комплексных ионов может быть использовано дл€ растворени€ труднорастворимых электролитов. онцентраци€ ионов в растворе определ€етс€ величиной произведени€ растворимости такого электролита. ƒобавл€€ в раствор вещества, образующие с одним из его ионов комплексное соединение, можно во многих случа€х достичь растворени€ осадка за счет комплексообразовани€. ƒобитьс€ этого тем легче, чем больше величина произведени€ растворимости и чем больше константа устойчивости комплексного иона. Ќапример, хлорид серебра AgCl раствор€етс€ в избытке аммиака, образу€ [Ag(NH3)2]Cl. ћенее растворимый AgI в аммиаке практически не растворим, но раствор€етс€ в тиосульфате натри€ Na2S2O3 по реакции

поскольку β[Ag(S2O3)2]3Ц на несколько пор€дков больше β[Ag(NH3)2]+.
омплексные ионы участвуют в реакци€х обмена с образованием более прочного или менее растворимого соединени€:

«десь M = Ni2+, Cu2+, Fe2+.






