| атион | –еактив | Ёффект | ”равнение реакции |
| Ag+ | KI | ∆елтый творожистый осадок, не растворим в NH4OH, но раствор€етс€ в Na2S2O3 | AgNO3+KI=AgI↓+KNO3 Ag++I--=AgI↓ AgI+2Na2S2O3= =Na3[Ag(S2O3)2]+NaI AgI+3Na++2S2O32--= =Na3[Ag(S2O3)2]+I-- |
| Ag+ | K2CrO4 | ирпично-красный осадок растворим в HNO3, NH4OH, трудно-растворим в уксусной кислоте | 2AgNO3+K2CrO4= =Ag2CrO4↓+2KNO3 2Ag++CrO42--=Ag2CrO4↓ |
| NaOH | „ерный осадок растворим в HNO3 при нагревании | 2AgNO3+2NaOH= =Ag2O↓+H2O+2NaNO3 2Ag++2OH--=Ag2O↓+H2O | |
| Na2S2O3 | Ѕелый осадок (растворим в избытке реактива) быстро переход€щий в желтый, черный осадок | 2AgNO3+Na2S2O3= =Ag2S2O3↓+2NaNO3 Ag2S2O3=Ag2SO3↓+S↓ Ag2SO3+S+H2O= =Ag2S↓+H2SO4 Ag2S2O3+3Na2S2O3(изб)= =2Na3[Ag(S2O3)2] | |
| –еакци€ се-ребр€ного зеркала (реакци€ среды Ц слабощело-чна€) | AgNO3+2NH4OH= =[Ag(NH3)2]NO3+2H2O 2[Ag(NH3)2]NO3+ +H2CO+H2O=2Ag↓+ +NH3↑+HCOONH4+ +NH4NO3 | ||
| Pb2+ | KI (реакци€ среды Ц слабокис-ла€) | ∆елтый осадок (+уксусна€ кислота + вода, нагреть до растворени€ осадка, при резком охлаждении раствора под струей холодной воды выпадает осадок в виде золотых чешуек) | Pb(NO3)2+2KI= =PbI2↓+2KNO3 Pb2+ +2I--=PbI2↓ |
| K2CrO4 | ∆елтый осадок ма-лорастворим в HNO3 и уксусной кислоте, но раствор€етс€ в щелочах | Pb(NO3)2+K2CrO4= =PbCrO4↓+2KNO3 Pb2+ +CrO42--=PbCrO4↓ | |
| Pb2+ | H2SO4 | Ѕелый осадок, растворим при нагревании в растворе щелочей | Pb(NO3)2+H2SO4= =PbSO4↓+2HNO3 Pb2+ +SO42--=PbSO4↓ PbSO4+4NaOH= =Na2PbO2+H2SO4+2H2O |
| NaOH | Ѕелый осадок растворим в кислотах и щелочах | Pb(NO3)2+2NaOH= =Pb(OH)2↓+2NaNO3 Pb2+ +2OH--=Pb(OH)2↓ | |
| Na2S | „ерный осадок растворим в HNO3 | Pb(CH3COO)2+Na2S= =PbS↓+2CH3COONa Pb2+ +S2--=PbS↓ 3PbS+8HNO3= =3Pb(NO3)2+3S↓+ +2NO↑+4H2O | |
| Hg22+ | –аствор дитизона в хлорофор-ме | —лой хлороформа окрашиваетс€ в красный цвет | |
| KI | √р€зно-зеленый осадок, растворим в избытке реактива | Hg2Cl2+2KI=Hg2I2↓+2KCl Hg22++2I--=Hg2I2↓ Hg2I2+2KI (изб) =K2[HgI4]+Hg↓ | |
| K2CrO4 | расно-бурый осадок, растворим в концен-трированной HNO3 и не растворим в щелочах | Hg2Cl2+K2CrO4= =Hg2CrO4↓+2KCl Hg22++CrO42--=Hg2CrO4↓ | |
| Hg22+ | NaOH | „ерный осадок | Hg2Cl2+2NaOH= =Hg2O↓+2NaCl+H2O Hg22++2OH--=Hg2O↓+H2O |
| ƒифенил-карбазид | —иний осадок или синее окрашивание |
¬опросы дл€ самопроверки
1. Ќазовите катионы, относ€щиес€ ко второй аналитической группе, групповой реактив. ќхарактеризуйте растворимость осадков.
2. акой цвет имеют осадки, образованные катионами второй группы с K2CrO4? KI? NaOH? Ќапишите уравнени€ реакций.
|
|
|
3. ¬ какой цвет окрашиваетс€ слой хлороформа, при взаимодействии катионов Ag+ и Pb2+ с раствором дитизона в хлороформе?
4. акой осадок образуетс€ при взаимодействии Hg22+ и дифенилкарбазида?
—водна€ таблица на реакции катионов второй аналитической группы
| –еактив | Ag+ | Pb2+ | Hg22+ |
| Cl-- | AgCl Ц белый творожистый осадок, раство-римый в растворе аммиака, Na2S2O3, (NH4)2CO3 | PbCl2 Ц белый хлопьевидный осадок, раст-воримый в гор€чей воде | Hg2Cl2 Ц белый осадок, при растворении в растворе аммиака выпадает черный осадок |
| I-- | AgI Ц желтый творожистый осадок, не раст-ворим в растворе аммиака, растворим в Na2S2O3 | PbI2 Цжелтый осадок | Hg2I2 - зеленоватый осадок, растворимый в избытке реактива |
| K2CrO4 | Ag2CrO4 Цкирпично-красный осадок, растворимый в азотной кислоте, растворе аммиака, трудно растворим в уксусной кислоте | PbCrO4 Ц желтый осадок, мало растворимый в азотной и уксусной кислотах, рас-творимый в щелочах | Hg2CrO4 Ц красно-бурый осадок, растворимый в концентрированной азотной кислоте, не растворимый в щелочах |
| NaOH | Ag2O Ц черный осадок, раство-римый в азотной кислоте при наг-ревании | Pb(OH)2 Ц белый осадок, раство-римый в кислотах и щелочах | Hg2O Ц черный осадок, растворимый в азотной кислоте |
| Na2S2O3 | Ѕелый осадок (Ag2S2O3), переход€щий в желтый (S), а затем в чер-ный(Ag2S) | ||
| KI | AgI Ц желтый осадок, не раст-воримый в NH4OH, растворимый в Na2S2O3 | PbI2 Ц золотисто-желтый осадок, растворимый в избытке KI | Hg2I2 Цзеленый осадок, растворим в избытке KI |
| H2SO4 | Ag2SO4 Ц белый осадок, раство-римый в гор€чей воде | PbSO4 Ц белый осадок, растворим в щелочах при нагревании | Hg2SO4 Ц белый осадок, растворимый в царской водке |
| Na2S | PbS Ц черный осадок, раст-воримый в азотной кислоте | ||
| –аствор дитизона в хлороформе | —лой хлороформа окраши-ваетс€ в желтый цвет | —лой хлороформа окрашиваетс€ в красный цвет | |
| ƒифенилкарбазид | —иний осадок или синее окрашивание |
—хема анализа смеси катионов первой и второй аналитических групп.
 |
 |
+HCl
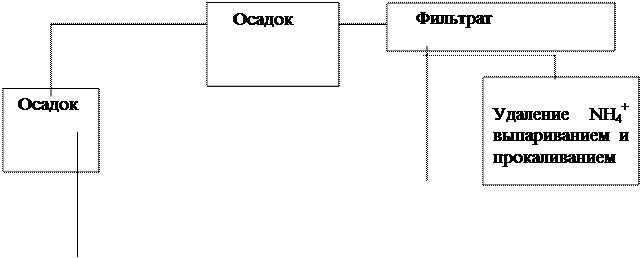

 гор€ча€ вода
гор€ча€ вода
| |||||||
 | |||||||
|
| ||||||
 |
| |||
 | |||
+NH4OH
| |||||
| |||||
 | |||||
|

+HNO3
|
¬ќѕ–ќ—џ ƒЋя «ј –≈ѕЋ≈Ќ»я
1) атионы первой аналитической группы. —войства катионов. –еактивы. ¬ыполнение качественных реакций. ”словие проведени€ реакций. –астворимость осадков.
2) јнализ смеси катионов.
3) атионы второй группы. јнализ смеси катионов первой и второй групп.






