≈сли записать объединенный газовый закон дл€ любой массы любого газа, то получаетс€ уравнение лапейрона-ћенделеева:
pV=(m/M)RT
где m - масса газа; M - молекул€рна€ масса; p Ц давление (при н.у.=101.3 кѕа); V - объем; T - абсолютна€ температура ( ); R - универсальна€ газова€ посто€нна€ (8,314 ƒж/(моль Х ) или 0,082 л атм/(моль Х )
ѕример 4
акой объем займет при температуре 17∞C и давлении 250 кѕа оксид углерода (II) массой 84 г?
–ешение.
ќтносительна€ плотность газов показывает, во сколько раз 1 моль одного газа т€желее (или легче) 1 мол€ другого газа.
—редн€€ молекул€рна€ масса смеси газов равна общей массе смеси, деленной на общее число молей:
ѕример1.
ѕлотность некоторого газообразного вещества по водороду равна 17. „ему равна его плотность по воздуху (ћср.=29).
–ешение.
DH2 =Mв-ва /MH2
ћв-ва=DH2*MH2 =34
Dвозд =Mв-ва /Mвозд. ср =34/29=1,17
ѕример5
ќпределите плотность по воздуху смеси азота, аргона и углекислого газа, если массовые доли компонентов составл€ли 15, 50 и 35% соответственно.
–ешение.
Dсмеси(повоздуху) =Mсмеси /Mвозд. =ћсмеси /29
Mсмеси = (15 Х 28 + 50 Х 40 + 35 Х 44) / 100 = (420 + 2000 + 1540) / 100 = 39,6
Dсмеси(повоздуху) =Mсмеси /29=39,6/29=1,37
«акон эквивалентов ».¬. –ихтер(1792-1794):
вещества реагируют и образуютс€ в эквивалентных количествах. Ёквивалентное соотношение означает одинаковое число моль эквивалентов. “.о. закон эквивалентов можно сформулировать иначе: число моль эквивалентов дл€ всех веществ, участвующих в реакции, одинаково.
Ёквивалент элемента Ц это такое его количество, которое соедин€етс€ с 1 молем водорода или замещает то же количество атомов водорода в химических реакци€х.
‘актор эквивалентности ƒэкв.(х) или 1/Z число, показывающее, кака€ реальна€ частица вещества ’ эквивалентна одному иону водорода в данной обменной реакции или одному электрону в данной окислительно-восстановительной реакции.
ƒэкв †рассчитывают на основании стехиометрических коэффициентов реакции.
Ќапример:
ƒл€ разных классов химических соединений существуют соответствующиеформулырасчета фактора эквивалентности. Ќапример, дл€ элемента он рассчитываетс€ так: 1/¬, где ¬ Ц валентность элемента в том или ином соединении. ¬от, например, основной оксид хрома Ц Cr2O3. ¬ этом соединении хром имеет валентность, равную 3. —ледовательно, его ƒэкв (фактор эквивалентности) равен 1/3. ј в бихромате кали€, имеющий формулу K2Cr2O7, уже валентность хрома равна 6, следовательно, его ƒэкв будет составл€ть 1/6.
≈сли речь идет о простом веществе, то есть таком, молекулы которого состо€т из атомов только одного элемента, то его фактор эквивалентности вычисл€етс€ по формуле 1/¬*N, где ¬ Ц валентность элемента, а N Ц количество его атомов в молекуле. Ћегко можноувидеть, что, например, кислород и озон, хоть в их состав входит только один элемент, будут иметь разные ƒэкв. ” кислорода, имеющего формулу молекулы ќ2, он будет равен 1/4, а у озона с формулой ќ3, соответственно, 1/6.
¬ уравнении реакции, например, реакции гидроксиданатри€с ортофосфорной кислотой. ¬ зависимости от того, в каких соотношени€х были вз€ты исходные вещества, могут образовыватьс€ разные продукты:
|
|
|
NaOH + H3PO4 = NaH2PO4 + H2O
2NaOH + H3PO4 = Na2HPO4 + 2H2O
3NaOH + H3PO4 = Na3PO4 + 3H2O
¬ первом случае на каждую молекулу щелочи, вступающую в реакцию, приходитс€ одна молекула кислоты. —ледовательно, фактор эквивалентности едкого натра равен 1, и фактор эквивалентности кислоты также равен 1.
¬о втором случае одна молекула кислоты вступает во взаимодействие с двум€ молекулами щелочи. “о есть на одну молекулу едкого натра приходитс€ 1/2 молекулы кислоты. “аким образом фактор эквивалентности щелочи по-прежнему равен 1, а фактор эквивалентности кислоты теперь равен 1/2.
—оответственно, в третьем случае, фактор эквивалентности едкого натра равен 1, а кислоты - 1/3, поскольку на одну молекулу щелочи приходитс€ три молекулы кислоты.
оличество моль - эквивалентов измер€етс€ в мол€х, как любое количество вещества. ћасса 1 моль эквивалента, мол€рна€ масса эквивалента, равна произведению фактора эквивалентности на мол€рную массу вещества
Mэкв= ƒэкв(х) ћ(х)
“ак, мол€рна€ масса эквивалента иона алюмини€
ћ экв (1/3јl+3) =1/3 27 = 9 г/моль
оличествомоль - эквивалентов можно определить по формуле
M
nэкв= ЧЧ††††††††††††
Mэкв
ћол€рна€ масса эквивалента и эквивалент элемента не €вл€ютс€ посто€нной величиной в соединении, а завис€т от валентности или степени окислени€ элемента.
Ќапример:††††††††††††††††††
ћэкв(—) в  = 12 ∙ 1/2 = 6 г/моль
= 12 ∙ 1/2 = 6 г/моль
ћэкв(—) в  ††= 12 ∙ 1/4 = 3 г/моль
††= 12 ∙ 1/4 = 3 г/моль
ѕосто€нными €вл€ютс€ эквиваленты ћэ(Ќ) = 1,008 г/моль ћэ(ќ) = 8 г/моль, ћэ (јl) = 9 г/моль, ћэ(—а) = 20 г/моль.
≈сли не учитывать конкретную химическую реакцию, то фактор эквивалентности и мол€рна€ масса эквивалентов сложных веществ рассчитываютс€ по формулам:
ћэ  =
=  ,
,
†где ћ Ц мол€рна€ масса вещества
†††† ¬ - валентность функциональной группы
N - число функциональных групп
†ƒл€ кислот функциональной группой €вл€етс€ ион водорода, дл€ оснований Ц ион гидроксила, дл€ солей Ц ион металла и т.д. (H+, OH-, Kat+n, An-n)†††
†ƒл€ определени€ эквивалентной массы сложного вещества в реакции следует разделить его полную массу (ћ) на сумму замещенных радикалов (H+, OH-, Kat+n, Ann-)
1) дл€ кислот: ƒэкв равен единице, деленной на основность кислоты, котора€ определ€етс€ числом ионов водорода
1/Z = ƒэкв(HCl) = 1
1/Z = ƒэкв(H2 SO4) = 1/2
1/Z = ƒэкв(H3 PO4) = 1/3
ћол€рна€ масса эквивалента кислоты равна произведению фактора эквивалентности на мол€рную массу кислоты
ћк
ћэкислоты = ƒэкв∙ ћк= Ч
Nн+
где: ћк - молекул€рна€ (формульна€) масса кислоты
Nн+ - число атомов водорода, способных замещатьс€ на металл.
†† ѕример: ћэ (H2SO4) = 1/2 ∙ 98 = 49 г/моль
1) дл€ оснований ƒэкв равен единице, деленной на кислотность, равную числу гидроксогрупп, вступающих в реакцию
ƒэкв(NaOH) =1/Z (NaOH) = 1
ƒэкв (Ca(OH)2) = 1/Z (Ca(OH)2) = 1/2
ћол€рна€ масса эквивалента основани€ равна произведению фактора эквивалентности на мол€рную массу основани€
ћэ основани€ = ƒэкв∙ ћосн
ѕример ћэ[Cr(OH)3] = 1/3*103 = 34,3 г/моль
2) дл€ солей: ƒэкв соли равен единице, деленной на произведение числа атомов металла в молекуле соли на степень окислени€ металла.
|
|
|
ƒэкв (Na2SO4) = 1/Z (Na2SO4) =1/2†††††††††††††††††††††††††††††††††††††††††††††††††††
ƒэквAl2(SO4)3 = 1/2 * 3†
ћол€рна€ масса эквивалента соли равна произведению фактора эквивалентности на мол€рную массу соли
ћэ соли = ƒэкв соли ∙ ћс 
ћэAl2(SO4)3= 57 г/моль
4) дл€ оксидов ƒэкв равен единице, деленной на произведение числа атомов кислорода на степень окислени€ кислорода
ƒэкв (H2O) = 1/Z (H2O) = 1/2
ƒэкв (SO2) = 1/Z (SO2) = 1/4
ƒэкв(Mn2O7) =1/7*2
ћол€рна€ масса эквивалента оксида равна произведению фактора эквивалентности на мол€рную массу оксида
ћэоксида = ƒэкв∙ ћокс
Ќапример:
ћэ (Mn2O7) =222/14 = 15,9 г/моль
¬ ќ¬– дл€ определени€ ƒэкв необходимо единицу разделить на число отданных или присоединенных электронов.
¬ещества вступают в реакцию в эквивалентных количествах:
†††††††††††††††††††††††† 2Ќ2 †+ ќ2 = 2Ќ2ќ
†††††††††††††††††††††††† 4экв 4экв4экв
¬ 1792 г. немецким физиком –ихтером был сформулирован закон эквивалентов: массы реагирующих друг с другом веществ (m1, m2) пропорциональны мол€рным массам их эквивалентов
»з математической записи закона эквивалентов следует, что количество моль - эквивалентов веществ в реакции равны между собой.
n экв1 = n экв2
ƒл€ реакций с участием газов используют мол€рный объем эквивалента Ц это объем, занимаемый 1 моль-эквивалентом газа.
“ак, ћЁ(Ќ) = 1г/моль, если моль газа 2г/моль занимают V=22,4л, то 1 эквивалент Ц в два раза меньший объем, равный 11,2 л.
VЁ ¬ (Ќ2) = 22,4: 2 = 11,2л.
јналогично ћЁ ¬(ќ)= ¼ ћ(ќ2) = 32: 4 = 8г/моль, отсюда Vэ(ќ2) = 22,4: 4 = 5,6л.
“аблицаЦ–асчет фактора эквивалентности
| „астица | ‘актор эквивалентности | ѕримеры |
| Ёлемент | 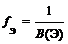 ,
√де ¬ (Ё) Ц валентность элемента ,
√де ¬ (Ё) Ц валентность элемента
| 

|
| ѕростое вещество |  ,
√де n (Ё) Ц число атомов элемента (индекс в химической формуле), ¬ (Ё) Ц валентность элемента ,
√де n (Ё) Ц число атомов элемента (индекс в химической формуле), ¬ (Ё) Ц валентность элемента
| f Ё(H2) = 1/(2×1) = 1/2; f Ё(O2) = 1/(2×2) = 1/4; f Ё(Cl2) = 1/(2×1) = 1/2; f Ё(O3) = 1/(3×2) = 1/6 |
| ќксид |  ,
√де n (Ё) Ц число атомов элемента (индекс в химической формуле оксида), ¬ (Ё) Ц валентность элемента ,
√де n (Ё) Ц число атомов элемента (индекс в химической формуле оксида), ¬ (Ё) Ц валентность элемента
| f Ё(Cr2O3) = 1/(2×3) = 1/6; f Ё(CrO) = 1/(1×2) = 1/2; f Ё(H2O) = 1/(2×1) = 1/2; f Ё(P2O5) = 1/(2×5) = 1/10 |
| ислота | 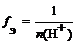 ,
√де n (H+) Ц число отданных в ходе реакции ионов водорода (основность кислоты) ,
√де n (H+) Ц число отданных в ходе реакции ионов водорода (основность кислоты)
| f Ё(H2SO4) = 1/1 = 1 (основность равна 1) или f Ё(H2SO4) = 1/2 (основность равна 2) |
| ќснование | 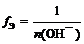 ,
√де n (ќHЦ) Ц число отданных в ходе реакции гидроксид-ионов (кислотность основани€) ,
√де n (ќHЦ) Ц число отданных в ходе реакции гидроксид-ионов (кислотность основани€)
| f Ё(Cu(OH)2) = 1/1 = 1 (кислотность равна 1) или f Ё(Cu(OH)2) = 1/2 (кислотность равна 2) |
| —оль |  ,
√де n (ће) Ц число атомов металла (индекс в химической формуле соли), ¬ (ће) Ц валентность металла; n (ј) Ц число кислотных остатков, ¬ (ј) Ц валентность кислотного остатка ,
√де n (ће) Ц число атомов металла (индекс в химической формуле соли), ¬ (ће) Ц валентность металла; n (ј) Ц число кислотных остатков, ¬ (ј) Ц валентность кислотного остатка
| f Ё(Cr2(SO4)3) = 1/(2×3) = 1/6(расчет по металлу) или f Ё(Cr2(SO4)3) = 1/(3×2) = 1/6(расчет по кислотному остатку) |
| „астица в окислительно-восстано≠вительных реакци€х |  ,
√де ,
√де  Ц число электронов, участвующих в процессе окислени€ или восстановлени€ Ц число электронов, участвующих в процессе окислени€ или восстановлени€
| Fe2++ 2  =Fe0
f Ё(Fe2+) =1/2;
MnO4Ц+ 8H++ 5 =Fe0
f Ё(Fe2+) =1/2;
MnO4Ц+ 8H++ 5  =Mn2++ 4H2O
f Ё(MnO4Ц) = 1/5 =Mn2++ 4H2O
f Ё(MnO4Ц) = 1/5
|
| »он |  ,
√де z Ц зар€д иона ,
√де z Ц зар€д иона
| f Ё(SO42Ц) = 1/2 |
ѕример. ќпределите фактор эквивалентности и эквивалент у солей: а) ZnCl2, б) Ќ—ќ3, в) (MgOH)2SO4.
–ешение: ƒл€ расчетов воспользуемс€ формулами, приведенными в таблице
а) ZnCl2 (средн€€ соль):
 .
.
f Ё(ZnCl2) = 1/2, поэтому эквивалентом ZnCl2€вл€етс€ частица ½ ZnCl2.
б) Ќ—ќ3(кисла€ соль):
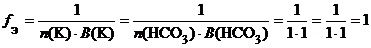 .
.
f Ё( Ќ—ќ3) = 1, поэтому эквивалентом Ќ—ќ3€вл€етс€ частица Ќ—ќ3.
в) (MgOH)2SO4 (основна€ соль):
 .
.
f Ё((MgOH)2SO4) = 1/2, поэтому эквивалентом(MgOH)2SO4€вл€етс€ частица 1/2(MgOH)2SO4.
Ёквивалент, как частица, может быть охарактеризован мол€рной массой (мол€рным объемом) и определеннымколичеством вещества n э. ћол€рна€ масса эквивалента (ћ Ё) Ц это масса одного моль эквивалента. ќна равна произведению мол€рной массы вещества на фактор эквивалентности:
|
|
|
| ћ Ё= ћ × f Ё. |
ћол€рна€ масса эквивалента имеет размерность Ђг/мольї.
ћол€рна€ масса эквивалента сложного вещества равна сумме мол€рных масс эквивалентов образующих его составных частей, например:
| ћ Ё(оксида) = ћ Ё(элемента) + ћ Ё(ќ) = ћ Ё(элемента) + 8 ћ Ё(кислоты) = ћ Ё(Ќ) + ћ Ё(кислотного остатка) = 1 + ћ Ё(кислотного остатка) ћ Ё(основани€) = ћ Ё(ће) + ћ Ё(ќЌ) = ћ Ё(ће) + 17 ћ Ё(соли) = ћ Ё(ће) + ћ Ё(кислотного остатка). |
√азообразные вещества помимо мол€рной массы эквивалента имеют мол€рный объем эквивалента ( или V Ё) Ц объем, занимаемый мол€рной массой эквивалента или объем одного моль эквивалента. –азмерность Ђл/мольї. ѕри н.у. получаем:
или V Ё) Ц объем, занимаемый мол€рной массой эквивалента или объем одного моль эквивалента. –азмерность Ђл/мольї. ѕри н.у. получаем:
ѕример 1: ћол€рна€ масса эквивалента некоторого металла равна 12 г/моль. ака€ масса этого металла прореагировала с кислотой, если при этом выделилось 1150 мл водорода (н.у.)?
–ешение: ѕо закону эквивалентов число моль эквивалентов прореагировавшего металла и водорода одинаково:
n экв (1/ z ће) = n экв (1/ z Ќ2).
¬ыразим число моль эквивалентов металла через отношение масс, а дл€ водорода Ц через отношение объемов:
 =
=  .
.
ћол€рный объем эквивалента водорода известен V(1/2 Ќ2) = 11.2 л/моль. “аким образом, в реакции с кислотой прореагировало металла
m(Me) =  =
=  = 1.23 г.
= 1.23 г.
ќтвет: 1.23 г.
ѕример 2: ѕри нагревании в кислороде 3.60 г некоторого металла его масса увеличилась до 5.04 г. –ассчитайте мол€рную массу эквивалента металла и его оксида.
–ешение: ѕо условию задачи металл реагирует с кислородом с образованием оксида (условное обозначение ћеќ). «акон эквивалентов дл€ данной реакции имеет вид:
n экв (1/ z ће) = n экв (1/ z ќ2) = n экв (1/ z ћеќ).
–ассчитаем сначала мол€рную массу эквивалента металла через отношение к кислороду (т.к. дл€ кислорода известна мол€рна€ масса эквивалента ћ(1/2 ќ)=8 г/моль).
 =
=  ;
;  =
= 
ќтсюда мол€рна€ масса эквивалента металла равна ћ(1/z ће) = 20 г/моль.
јналогично произведем расчет дл€ оксида:
 =
=  ;
; 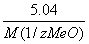 =
=  .
.
ћ(1/z ћеќ) = 28 г/моль.
ƒругим вариантом расчета мол€рной массы эквивалента оксида будет использование формулы
ћ(1/z ћеќ) = ћ(1/z ће) + 8 = 28 г/моль.
ќтвет: 20 г/моль; 28 г/моль.
ѕример 3: ¬ оксиде некоторого элемента массова€ дол€ кислорода составл€ет 20 %. –ассчитайте мол€рную массу эквивалента элемента и его оксида.
–ешение: ѕро любой оксид можно сказать, что он получен по реакции окислени€ элемента кислородом. «акон эквивалентов дл€ такой реакции окислени€ можно записать как:
n экв (1/ z элемента) = n экв (1/ z ќ2) = n экв (1/ z оксида);
 =
=  .
.
ƒалее необходимо рассчитать массы элемента и кислорода в любой произвольно вз€той массе оксида. Ќаиболее удобно прин€ть массу оксида за 100 г (100%).“.о., в 100 г оксида содержитс€ 20 г кислорода и 80 г элемента.
 =
= 
ћол€рна€ масса эквивалента элемента равна ћ(1/z элемента) = 32 г/моль. ќтсюда мол€рна€ масса эквивалента оксида составл€ет:
ћ(1/z оксида) = ћ(1/z элемента) + 8 = 32 + 8 = 40 г/моль.
ќтвет: 32 г/моль; 40 г/моль.






