—амопроизвольному протеканию химических процессов в изобарно-изотермических услови€х способствуют уменьшение энтальпии и увеличение энтропии системы. ѕерва€ тенденци€ характеризуетс€ энтальпийным фактором и выражаетс€ через D Ќ, а втора€ - характеризуетс€ энтропийным фактором и количественно выражаетс€ через T × D S.
—овместное вли€ние энтальпийного и энтропийного факторов учитывает изменение энергии √иббса D G ( )
)
D G = D Ќ - T × D S,
где “ - абсолютна€ температура, .
—амопроизвольно при –,“ = const в пр€мом направлении могут протекать реакции, дл€ которых D G < 0. ≈сли D G реакции больше нул€, то такой процесс может протекать самопроизвольно только в обратном направлении. –еакции, имеющие D G = 0, наход€тс€ в состо€нии равновеси€, то есть дл€ них равноверо€тно пр€мое и обратное течение процесса.
–асчет изменени€ энергии √иббса реакции в стандартных услови€х D G  †можно осуществить двум€ способами:
†можно осуществить двум€ способами:
1. ѕо уравнению D G  †= D Ќ
†= D Ќ  †- “ × D S
†- “ × D S  †. –асчет D Ќ
†. –асчет D Ќ  †и D S
†и D S  †подробно рассмотрен ранее.
†подробно рассмотрен ранее.
2. ѕо формуле, аналогичной при расчете D Ќ  †реакции через энтальпии образовани€ участников реакции. ¬ этом случае дл€ реакции вида
†реакции через энтальпии образовани€ участников реакции. ¬ этом случае дл€ реакции вида
a A + b B = d D + f F
D G  ††= d D G
††= d D G 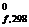 (D) + f D G
(D) + f D G 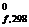 (F) - a D G
(F) - a D G 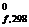 (A) - b D G
(A) - b D G 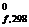 (B),
(B),
где D G 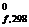 †- стандартна€ энерги€ √иббса образовани€ вещества,
†- стандартна€ энерги€ √иббса образовани€ вещества,  .
.
—тандартной энергией √иббса образовани€ вещества D G 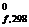 †называют изменение энергии √иббса реакции образовани€ 1 моль соединени€ из простых веществ, наход€щихс€ в стандартных услови€х. D G
†называют изменение энергии √иббса реакции образовани€ 1 моль соединени€ из простых веществ, наход€щихс€ в стандартных услови€х. D G 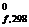 †простых веществ равны нулю, а дл€ сложных веществ - определены и содержатьс€ в справочной литературе (см. приложение).
†простых веществ равны нулю, а дл€ сложных веществ - определены и содержатьс€ в справочной литературе (см. приложение).
Ќаправление химической реакции, прежде всего, определ€етс€ ее природой. “ак, дл€ экзотермических реакций (D Ќ < 0), сопровождающихс€ возрастанием числа моль газообразных веществ (D S > 0), при любой температуре изменение энергии √иббса будет отрицательным. “о есть независимо от температуры такие реакции могут протекать только в пр€мом направлении. Ќаоборот, эндотермические реакции (D Ќ > 0), сопровождающихс€ уменьшением числа моль газообразных веществ (D S < 0), независимо от значени€ температуры в системе могут протекать только в обратном направлении, так как в этом случае D G всегда положительное.
ѕри сочетании D Ќ > 0, D S > 0 или D Ќ < 0, D S < 0 возможность протекани€ реакции в пр€мом направлении будет зависеть от температуры. ѕри невысоких температурах  , поэтому энтальпийный фактор вносит больший вклад в значение D G. ѕри высоких температурах, когда
, поэтому энтальпийный фактор вносит больший вклад в значение D G. ѕри высоких температурах, когда  , знак D G будет определ€тьс€ знаком энтропийного фактора.
, знак D G будет определ€тьс€ знаком энтропийного фактора.
ѕример 1. ¬ычислите D G  †реакции двум€ способами 2SO2 + ќ2 = 2Sќ3.
†реакции двум€ способами 2SO2 + ќ2 = 2Sќ3.
– е ш е н и е
1. ¬ычислим D G  , использу€ справочные данные о D G
, использу€ справочные данные о D G 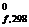 :
:
D G  ††= 2D G
††= 2D G 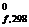 (SO3) - 2D G
(SO3) - 2D G 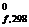 (SO2) - D G
(SO2) - D G 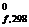 (O2) =
(O2) =
= 2(-371,17) - 2(-300,21) - 0 = -141,92 кƒж.
2. ¬ычислим D G  , использу€ справочные данные об энтальпии образовани€ и энтропии веществ:
, использу€ справочные данные об энтальпии образовани€ и энтропии веществ:
D S  ††= 2 S
††= 2 S  (SO3) - S
(SO3) - S  (O2) - 2 S
(O2) - 2 S  (SO2) = 2×256,7 - 205,0 -2×248,1 = -187,8
(SO2) = 2×256,7 - 205,0 -2×248,1 = -187,8  ;
;
|
|
|
D Ќ  †= 2D Ќ
†= 2D Ќ 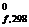 (Sќ3) - 2D Ќ
(Sќ3) - 2D Ќ 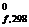 (SO2) = 2(-395,9) - 2(-296,9) = -198,0 кƒж;
(SO2) = 2(-395,9) - 2(-296,9) = -198,0 кƒж;
D G  †= D Ќ
†= D Ќ  †- “ × D S
†- “ × D S  †= -198000 - 298×(-187,8) = -142036 ƒж = -142,0 кƒж.
†= -198000 - 298×(-187,8) = -142036 ƒж = -142,0 кƒж.
ѕример 2. ќпределите знак D G, не прибега€ к расчетам дл€ реакции
—аќт + —ќ2, г = —а—ќ3, т; D Ќ  †= -178,1 кƒж/моль.
†= -178,1 кƒж/моль.
– е ш е н и е
–еакци€ экзотермическа€, так как D Ќ  †< 0. ѕротекает с уменьшением числа моль газообразных веществ, следовательно, D S
†< 0. ѕротекает с уменьшением числа моль газообразных веществ, следовательно, D S  †< 0. “огда при невысоких температурах
†< 0. “огда при невысоких температурах  , а знак D G
, а знак D G  †отрицательный (реакци€ протекает в пр€мом направлении). ѕри высоких температурах
†отрицательный (реакци€ протекает в пр€мом направлении). ѕри высоких температурах  , следовательно, D G
, следовательно, D G  †положительное.
†положительное.
ѕример 3. ќпределите температуру равноверо€тного протекани€ реакции в пр€мом и обратном направлении, если D Ќ = -235 кƒж, а D S = -95  .
.
– е ш е н и е
ѕри равноверо€тном протекании реакции в пр€мом и обратном направлении D G = 0. “огда † D Ќ - T × D S = 0. ќтсюда
T =  †
†  K.
K.
« ј ƒ ј „ »
1. ¬ каком направлении при стандартных услови€х будет протекать реакци€ N2, г + ќ2, г = 2NO г? ќтвет подтвердите расчетами.
2. ¬ каком направлении при стандартных услови€х будет протекать реакци€ N2ќ г + Nќ2, г = 3NO г? ќтвет подтвердите расчетами.
3. ¬ каком направлении при стандартных услови€х будет протекать реакци€ N2O г + Nќ = NO2,г + N2, г? ќтвет подтвердите расчетами.
4. ћожно ли при стандартных услови€х восстановить водородом оксиды CuO и PbO? ќтвет объ€сните.
5. ¬озможно ли получение свинца из оксида свинца (II) путем его восстановлени€ графитом и при какой температуре?
6. ¬ычислите изменение энергии √иббса реакции при 1000 , если D Ќ  = 131,3 кƒж, а D S
= 131,3 кƒж, а D S  †= 133,6 ƒж/ (вли€нием “ †на D Ќ и D S пренебречь).
†= 133,6 ƒж/ (вли€нием “ †на D Ќ и D S пренебречь).
7. ¬ычислите изменение D G реакции при 298 2Ќ2ќг + —гр = 2Ќ2 + —ќ2. ƒействием какого фактора - энтальпийного или энтропийного - определ€етс€ знак D G данной реакции?
8. ¬ычислите D S  , D Ќ
, D Ќ  †и D G
†и D G  †дл€ реакции NH4Clк = NH3, г + HClг. ак вли€ет температура на направление протекани€ данной реакции?
†дл€ реакции NH4Clк = NH3, г + HClг. ак вли€ет температура на направление протекани€ данной реакции?
9. ака€ из приведенных реакций термодинамически более веро€тна
1) 4NH3 + 3O2 = 2N2 + 6H2Oг; 2) 4NH3 + 5O2 = 4NO + 6H2Oг.
ќтвет подтвердите расчетами.
10. ќпределите направление самопроизвольного протекани€ реакции при стандартных услови€х SO2 + 2H2 = Sкр + 2H2Oг .
11. ¬ычислите изменение D G реакции при 298 ZnS +  O2 = ZnO + Sќ2. ƒействием какого фактора - энтальпийного или энтропийного - определ€етс€ знак D G данной реакции?
O2 = ZnO + Sќ2. ƒействием какого фактора - энтальпийного или энтропийного - определ€етс€ знак D G данной реакции?
12. ¬ычислите изменение энергии √иббса реакции при 1500 , если D Ќ  = 221,0 кƒж, а D S
= 221,0 кƒж, а D S  †= 53,2 ƒж/ (вли€нием “ †на D Ќ и D S пренебречь).
†= 53,2 ƒж/ (вли€нием “ †на D Ќ и D S пренебречь).
13. ќпределите температуру равноверо€тного протекани€ реакции в пр€мом и обратном направлении, если D Ќ = 138 кƒж, а D S = 105  .
.
14. ака€ из приведенных реакций термодинамически более веро€тна
1) N2 + O2 = 2Nќ; 2) N2 + 2O2 = 2NO2.
ќтвет подтвердите расчетами.
15. ¬ычислите D S  , D Ќ
, D Ќ  †и D G
†и D G  †дл€ реакции —ќ2 = —гр + ќ2. ак вли€ет температура на направление протекани€ данной реакции?
†дл€ реакции —ќ2 = —гр + ќ2. ак вли€ет температура на направление протекани€ данной реакции?
16. ¬озможно ли получение цинка из оксида цинка (II) путем его восстановлени€ графитом и при какой температуре?
17. ѕри какой температуре начнетс€ реакци€ PbOк + —ќ = Pbк + —ќ2? ¬ли€нием температуры на D Ќ и D S пренебречь.
18. ќпределите направление самопроизвольного протекани€ реакции при стандартных услови€х —O2 + 2H2 = —гр + 2H2Oг.
19. ¬ычислите изменение D G реакции при 298 FeS +  O2 = FeO + Sќ2. ƒействием какого фактора - энтальпийного или энтропийного - определ€етс€ знак D G данной реакции?
O2 = FeO + Sќ2. ƒействием какого фактора - энтальпийного или энтропийного - определ€етс€ знак D G данной реакции?
|
|
|
20. ¬ычислите D G  †и D G
†и D G  †дл€ реакции Ќ2ќг + —гр = —ќг + Ќ2, г. ак вли€ет температура на термодинамическую веро€тность протекани€ процесса в пр€мом направлении?
†дл€ реакции Ќ2ќг + —гр = —ќг + Ќ2, г. ак вли€ет температура на термодинамическую веро€тность протекани€ процесса в пр€мом направлении?
21. ћожно ли при стандартных услови€х восстановить водородом оксиды ZnO и NiO? ќтвет объ€сните.
22. ¬ычислите D G  †реакции двум€ способами 2Fe + Al2O3 = Fe2O3 + 2 Al.
†реакции двум€ способами 2Fe + Al2O3 = Fe2O3 + 2 Al.
23. ќпределите температуру равноверо€тного протекани€ реакции в пр€мом и обратном направлении, если D Ќ = 38 кƒж, а D S = 207  .
.
24. ¬ каком направлении при стандартных услови€х будет протекать реакци€ —Ќ4, г + 2ќ2, г = —ќ2, г + 2Ќ2ќж? ќтвет подтвердите расчетами.
25. ¬ычислите изменение энергии √иббса реакции при 2000 , если D Ќ  = 242,3 кƒж, а D S
= 242,3 кƒж, а D S  †= 195,6 ƒж/ (вли€нием “ †на D Ќ и D S пренебречь).
†= 195,6 ƒж/ (вли€нием “ †на D Ќ и D S пренебречь).
ѕ–»Ћќ∆≈Ќ»≈






