¬ растворе могут протекать одна или несколько побочных реакций с ионами осадка (образование малодиссоциированного соединени€, комплекса и т.д.).
Ќапример, равновесие в насыщенном растворе оксалата кальци€ в присутствии сол€ной кислоты HCl можно представить так:
CaC2O4тв ó Ca2+ + C2O42Ц
HCl ó H+ + ClЦ
ѕобочные (конкурентные) реакции C2O42Ц + H+ ó HC2O4Ц
HC2O4Ц + H+ ó H2C2O4
¬ этом случае дл€ описани€ равновеси€ между раствором и осадком используют условное произведение растворимости ѕ–у, которое выражаетс€ даже не через равновесные, а через общие или аналитические концентрации —.
ѕ–у = —јаЈ—¬в
»ли применительно к нашему случаю

ѕ–у = f (T, P, природы растворител€, ионной силы μ, мольных долей соответствующих ионов αј и α¬)
—в€зь с термодинамическим произведением растворимости:
ѕ–т = аја Ја¬в = [A]aЈ[B]bЈfAaЈfBb = —јаЈ—¬в fAaЈfBb αјЈα¬ = ѕ–уЈfAaЈfBb αјаЈα¬в
ѕоскольку вли€ние электростатических взаимодействий сказываетс€ на растворимости значительно слабее, чем химические взаимодействи€, то при расчете условного ѕ– сомножитель, состо€щий из коэффициентов активности fAaЈfBb, не учитывают.

»так, когда и каким ѕ– следует пользоватьс€?
1. ¬ реальной практике, если электролит мало растворим ѕ– < 10-7 и ионна€ сила раствора μ стремитс€ к 0, пользуютс€ ѕ–т.
2. μ≠0 (заметно растворимое соединение или присутствует посторонний сильный электролит). ѕользуютс€ ѕ–р.
3. ≈сли нельз€ пренебречь конкурентными реакци€ми, равновесие раствор-осадок описывают с помощью ѕ–у.
ѕод растворимостью S понимают способность веществ образовывать гомогенную систему с растворителем. –астворимость измер€ют в моль/л, г/100мл, г/мл и т.д. –астворимость осадков Ц важна€ величина, позвол€юща€ определ€ть концентрацию вещества в насыщенном растворе, рассчитать возможность образовани€ осадка и т.д.
≈сли осадок малорастворимого сильного электролита ј¬ состоит из ионов одинаковой зар€дности, тогда растворимость S Ц это равновесна€ концентраци€ иона ј или иона ¬. ≈сли обозначить эту концентрацию через x, то
ѕ– = [A]Ј[B] = x2
S = x = 
ƒл€ осадка электролита, состо€щего из ионов разной зар€дности ја¬в равновесие в насыщенном растворе
ја¬тв ó aA + в¬

ќтсюда [A] = aЈS и [B] = bЈS
ѕ– (ја¬в)= [A]aЈ[B]b = [aЈS]aЈ[ bЈS]b = aaЈbbЈSa+b. ќтсюда

Ќапример, растворимость хромата серебра Ag2CrO4 (ѕ– = 1,44Ј10-12) можно рассчитать
 = 7,5Ј10-5моль/л.
= 7,5Ј10-5моль/л.
„ем меньше растворимость, тем труднее раствор€етс€ электролит. Ќапример, BaSO4 (ѕ– = 1,05Ј10-10) с трудом при кип€чении раствор€етс€ только в концентрированной серной кислоте, в то врем€ как CaSO4 (ѕ– = 9,1Ј10-6) довольно хорошо растворим в воде Ц гипсова€ вода.
”словие выпадени€ осадка
„тобы ответить на вопрос, выпадет ли осадок в данных услови€х, необходимо рассчитать величину произведени€ концентраций ѕ и сравнить ее с табличной величиной ѕ–. ѕри этом возможны 3 случа€:
1. ѕ < ѕ–. “акой раствор называетс€ ненасыщенным. ќсадок в таком растворе не образуетс€. ћолекулы осадка сразу же распадаютс€ на ионы, т.к. их концентраци€ ниже равновесной.
|
|
|
2. ѕ = ѕ–. Ётот раствор называетс€ насыщенным. ¬ нем наступает подвижное равновесие. ќсадок не выпадает.
3. ѕ > ѕ–. ќсадок образуетс€ только в пересыщенном растворе. ќбразование осадка будет продолжатьс€ до наступлени€ равенства ѕ = ѕ– и превращени€ раствора из пересыщенного в насыщенный. Ќаступает равновесие и дальнейшее образование осадка прекращаетс€.
—войство насыщенного раствора сохран€ть посто€нным произведение активностей (концентраций) ионов в соответствующих степен€х называют правилом произведени€ растворимости.
ѕо этому правилу невозможно существование таких растворов, в которых произведение активностей превышало бы табличное значение ѕ– при данной температуре. ≈сли произведение активностей в соответствующих степен€х превышает ѕ–, то должно произойти образование осадка и концентраци€ ионов в растворе должна уменьшитс€ до таких значений, которые удовлетвор€ли бы правилу ѕ–.
¬ соответствии с правилом ѕ–, если концентраци€ (активность) одного из ионов, вход€щих в выражение ѕ–, увеличиваетс€, то концентраци€ (активность) другого уменьшаетс€.
Ёто действие одноименного иона лежит в основе методов количественного осаждени€ и используетс€ в аналитической химии.
–ассчитаем, например, растворимость AgCl (ѕ–т = 1,78Ј10-10). в воде и в 0,01ћ растворе KCl:
1) S = 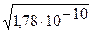 = 1,3Ј10-5 M
= 1,3Ј10-5 M
2) ≈сли обозначить равновесную концентрацию ионов серебра и хлорид-ионов через х, то в 0,01ћ растворе KCl произведение растворимости хлорида серебра можно записать ѕ– = x Ј(x + 0,01).
ќтсюда  . ѕренебрежем x в знаменателе как слагаемым. ќтсюда S = [Ag+] = x = 1,78Ј10-10 / 0,01 =1,78Ј10-8. ¬идно, что одноименный ион оказывает значительно более существенное вли€ние на растворимость, чем ионна€ сила. ѕри этом растворимость снижаетс€ в тыс€чу раз.
. ѕренебрежем x в знаменателе как слагаемым. ќтсюда S = [Ag+] = x = 1,78Ј10-10 / 0,01 =1,78Ј10-8. ¬идно, что одноименный ион оказывает значительно более существенное вли€ние на растворимость, чем ионна€ сила. ѕри этом растворимость снижаетс€ в тыс€чу раз.






