оличественный анализ Ц заключаетс€ в определении количественного содержани€ отдельных составных частей сложного вещества
√равиметрический анализ основан на точном измерении массы определ€емого вещества в виде соединени€ или простого вещества определенного состава. ќсновным инструментом €вл€ютс€ весы.
√равиметрические методы подраздел€ютс€ на две подгруппы:
I. методы осаждени€
II. методы отгонки.
¬ методах осаждени€ навеску анализируемого вещества перевод€т в раствор, после этого определ€емый элемент осаждают в виде малорастворимого соединени€. ¬ыпавший осадок отдел€ют фильтрованием, тщательно промывают или высушивают, и точно взвешивают. ѕо массе осадка и его формуле рассчитывают содержание определенного элемента в % по массе.
¬ методах отгонки определ€емый компонент удал€ют в виде летучих продуктов, и по убыли в весе суд€т о содержании элемента.
“ребовани€ к осадкам:
ќсаждаемой формой Ц называют то соединение, которое образуетс€ при взаимодействии с реагентом Ц осадителем, а весовой формой Ц соединение, которое взвешивают дл€ получени€ окончательного результата анализа.
Ќапример, при определении кремни€ в чугунах формой осаждени€ €вл€етс€ кремниева€ кислота H2SiO3ЈnH2O, а весовой формой €вл€етс€ безводна€ двуокись кремни€, получающа€с€ в результате прокаливани€ при температуре около 1000о—. иногда осаждаема€ и весова€ форма могут представл€ть собой одно и тоже соединение. Ќапример, при определении серы весовым методом ее осаждают из раствора, и взвешивают в виде сульфата бари€, который при прокаливании химически не измен€етс€.
Ётапы в гравиметрическом методе анализа:
1. ¬з€тие точной навески вещества (минерала, сложного материала...)
2. ѕеревод (растворение) твердой навески в растворимое состо€ние тем или иным способом.
3. ќсаждение определ€емого компонента в виде малорастворимого соединени€, которое выдел€ют из раствора.
4. ‘ильтрование.
5. ѕромывание
6. ¬ысушивание (и | или прокаливание)
7. ќхлаждение.
8. ¬звешивание на аналитических весах.
9. ¬ычисление количества определ€емого компонента по массе взвешенного вещества
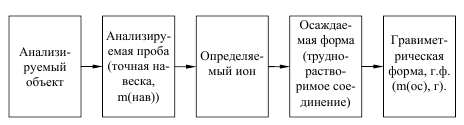
ќбща€ схема определени€ по методу осаждени€:
√равиметрический фактор (F) Ц отношение молекул€рной массы определ€емого вещества к мол€рной массе гравиметрической формы с коэффициентами a и b, на которые нужно умножить это отношение, чтобы молекул€рные массы были эквивалентны друг другу:
F = а ћ(опр.в-ва) / bћ(г.ф.).
а, b Ц стехиометрические коэффициенты; ћ(опр.в-ва) Ц молекул€рна€ масса определ€емого вещества, г; ћ(г.ф.). Ц молекул€рна€ масса гравиметрической
формы.
1. –асчет навески анализируемого вещества g:
а) дл€ кристаллических осадков:
g = х ×ћ (опр.в-ва)×0,5/ у× ћ(г.ф.)
б) дл€ аморфных осадков
g = х ×ћ (опр.в-ва)×0,1/ у× ћ(г.ф.)
где g Ц навеска пробы, г; х и у Ц стехиометрические коэффициенты в уравнени€х реакций перехода определ€емого вещества (х) в форму осаждени€ и далее в
весовую гравиметрическую форму (у); коэффициенты 0,5 и 0,1 Ц рекомендуемые массы весовой формы (г) соответственно дл€ кристаллических и аморфных осадков; ћ (опр.в-ва) Ц молекул€рна€ масса- определ€емого вещества; ћ(г.ф.)
|
|
|
Ц молекул€рна€ масса гравиметрической формы.
2. –асчет объема раствора реактива-осадител€ Vр, производ€т по формуле:
Vр =1,5 × х ×ћo× g×100/ у ×ћ (опр.в-ва) × с ×ρ
где 1,5 Ц коэффициент, показывающий, во сколько раз больше необходимо вз€ть осадител€; х и у Ц стехиометрические коэффициенты в уравнении реакции осаждени€ соответственно дл€ реактива-осадител€ и определ€емого вещества; ћo и ћ (опр.в-ва) Цмолекул€рные массы соответственно реактива-осадител€ и
определ€емого вещества; g Ц навеска определ€емого вещества, г; с Ц массова€ дол€ осадител€ в растворе, %; ρ Ц плотность раствора-осадител€, г/см3.
3. –асчет результатов анализа:
x = mF 100/ g;
где х Ц массова€ дол€ определ€емого вещества, %; m Ц масса весовой
гравиметрической формы, г; F Ц гравиметрический фактор; g Ц масса навески, г.
ѕ–»ћ≈–џ –≈Ў≈Ќ»я «јƒј„
ѕример 1. –ассчитать гравиметрический фактор при определении кали€ по схеме: K→ K2PtCl6→ Pt
–ешение: Ќаходим стехиометрическое соотношение между соединени€ми схемы:
2K(OB)→ K2PtCl6→ Pt(√‘)
ћ (опр.в-ва) = ћ K= 39; а = 2;
ћ(г.ф.). = 195; b= 1
F= 2 ∙ 39/1 ∙ 195 =0,400
ѕример 2. –ассчитать навеску Fe(NO3)3∙ 6Ќ2ќ, необходимую дл€ определени€ железа в виде Fe2ќ3.
”равнени€ реакций, протекающих при анализе:
Fе3+ + 3ќЌ-→ Fе(ќЌ)3
2 Fе(ќЌ)3 → Fe2ќ3+ 3Ќ2ќ т. е.
2 Fe(NO3)3(OB)→2 Fе(ќЌ)3→ Fe2ќ3(√‘)
ћ (опр.в-ва) = ћ Fe(NO3)3∙ 6Ќ2ќ = 350; а = 2;
ћ(г.ф.) = ћ Fe2ќ3= 160; b = 1;
»спользуем формулу дл€ расчетанавески анализируемого вещества
а) дл€ кристаллических осадков:
g = х ×ћ (опр.в-ва)×0,5/ у× ћ(г.ф.)
б) дл€ аморфных осадков
g = х ×ћ (опр.в-ва)×0,1/ у× ћ(г.ф.)
где g Ц навеска пробы, г; х и у Ц стехиометрические коэффициенты в уравнени€х реакций перехода определ€емого вещества (х) в форму осаждени€ и далее в
весовую гравиметрическую форму (у); коэффициенты 0,5 и 0,1 Ц рекомендуемые массы весовой формы (г) соответственно дл€ кристаллических и аморфных осадков; ћ (опр.в-ва) Ц молекул€рна€ масса- определ€емого вещества; ћ(г.ф.)
Ц молекул€рна€ масса гравиметрической формы.
g =2 ×350× 0,1/1× 160=0, 4375г.
ѕример 2. –ассчитать навеску мрамора, необходимую дл€ определени€ кальци€ в виде —аќ.
”равнени€ реакций, протекающих при анализе:
—а—ќ3 + 2Ќ+ →—а2+ + Ќ2ќ+ —ќ2↑
—а2+ + Ќ2—2ќ4→ —а—2ќ4 + 2Ќ+
—а—2ќ4→ —аќ+—ќ↑+—ќ2↑
—а—ќ3 (OB)→ —а2+→ —а—2ќ4 → —аќ (√‘)
“ак как одна молекула —а—ќ3 в ходе анализа переходит в одну молекулу —аќ, то в уравнении дл€ расчета гравиметрического фактора а = b = 1. ќтсюда:
ћ (опр.в-ва) =M —а—ќз =100; а = 1
ћ(г.ф.) =M —аќ = 56; b = 1
g =1 ×100× 0,5/1× 56=0,8927г.
ѕример 3. –ассчитать объем раствора NH4OH с массовой долей аммиака 10,4% (плотность Ц 0,956 г/см3), необходимый дл€ осаждени€ алюмини€ из навески AlCl3 массой 0,5 г.
”равнение реакции осаждени€:
AlCl3 + 3NH4ќЌ → Al(ќЌ)3 + 3NH4Cl.
»з этого уравнени€ видно, что дл€ осаждени€ одной молекулы AlCl3
|
|
|
необходимо три молекулы NH4Cl, т. е. а=3, b= 1. Ќаходим молекул€рную массу
веществ.
V= 1,5× 3× 35,05× 0,5000×100/1×133,34×10,4×0,956 = 5,95 мл.
«адачи
121. ѕри определении алюмини€ массой 0,010 г один студент использовал гравиметрический метод, основанный на осаждении аммиаком, другой Ц метод, основанный на осаждении оксихинолином. ¬ каком случае можно ожидать более точный результат?
122. акой из методов более точен и почему: а) метод, основанный на осаждении гидроксида никел€; б) метод, основанный на осаждении диметилглиоксимата никел€?
123. акие преимущества имеет гомогенный осадитель —ќ(NH2)2 по сравнению с аммиаком при осаждении гидроксида железа (III)?
124. ¬ каком случае образуетс€ более чистый крупнокристаллический осадок сульфата бари€ по сравнению с осадком, полученным при осаждении серной кислотой?
125. акой реагент Ц K2C2O4, Na2C2O4, H2C2O4 или (NH4)2C2O4 Ц целесообразно использовать при осаждении оксалата кальци€?
126. акие требовани€ предъ€вл€ютс€ к осаждаемой и гравиметрической формам?
127. ќт каких факторов завис€т размер и число частиц осадка?
128. акие требовани€ предъ€вл€ютс€ к осадителю в гравиметрическом анализе?
129. акими преимуществами обладают органические осадители перед неорганическими? ѕриведите примеры органических осадителей.
130. »з навески цемента массой 1,500 г получили 0,2105 г пирофосфата магни€. ¬ычислить массовую долю (%) оксида магни€ в цементе. ќтвет: 5,08%.
131. акую массу Fe3O4 следует вз€ть дл€ получени€ 0,200 г Fe2O3. ќтвет: 0,19 г.
132. ¬ычислить гравиметрический фактор дл€ вычислени€ массы HF, определ€емого по схеме: HF → CaF2 → CaSO4. ќтвет: 0,2939.
133. ¬ычислить гравиметрический фактор дл€ вычислени€ массы мышь€ка, определ€емого по схеме: As → As2S3 → SO42- → BaSO4. ќтвет: 0,2140.
134. ¬ычислить гравиметрический фактор дл€ вычислени€ массы —а—2, определ€емого по схеме: CaC2 → H2C2 → Ag2C2 → AgCl. ќтвет: 0,2236.
135. »з навески 1,225 г суперфосфата получили прокаленный осадок CaSO4 массой 0,3756 г. ¬ычислить массовую долю (%) Ca3(PO4)2 в суперфосфате. ќтвет: 23,29%.
136. »з раствора хлорида магни€ получили осадок оксихинолината магни€ Mg(C9H6ON)2 массой 0,2872 г. —колько граммов магни€ содержитс€ в исследуемом растворе? ќтвет: 0,0223 г.
137. “ехнический хлорид бари€ содержит около 97% BaCl2Ј2 H2O. акую навеску его следует вз€ть дл€ получени€ 0,300 г осадка BaSO4. ќтвет: 0,320 г.
138. акой объем сол€ной кислоты (ρ= 1,17 г/см3) потребуетс€ дл€ осаждени€ серебра в виде AgCl из 2,0 г сплава, содержащегос€ 22 % Ag, при использовании полуторного избытка осадител€? ќтвет: 0,56 мл.
139. акой объем Ќ2SO4 (ρ = 1,24 г/см3) потребуетс€ дл€ превращени€ 0,350 г —аќ в —аSO4? ќтвет: 1,5 мл.
140. ¬ычислить массовую долю (%) Ag в сплаве, если из навески сплава массой 0,2466 г после соответствующей обработки получили 0,2675 г хлорида серебра. ќтвет: 81,64%.
“итриметрический анализ
“итриметрический анализ основан на точном измерении объемов веществ, вступающих в химическую реакцию. ¬ этом методе используют растворы реактивов точно известной концентрации Ц титранты.
“итрование Ц процесс медленного прибавлени€ титранта к раствору определ€емого вещества. ћомент титровани€, когда количество прибавленного титранта становитс€ эквивалентным количеству определ€емого вещества, называетс€ эквивалентной точкой титровани€ или точкой эквивалентности. ≈е определ€ют с помощью индикаторов или по изменению физико-химических характеристик титруемого раствора. “итриметрический анализ отличаетс€ быстротой и точностью полученных результатов. “очность вычислений до 4-х значащих цифр после зап€той обуславливаетс€ возможност€ми аналитических весов и примен€емой дл€ анализа величиной навески вещества (0,1ч1,0 г).
ѕо типу реакции, используемой при титровании, различают
Ц кислотно-основное, окислительно-восстановительное,
|
|
|
Ц осадительное
Ц комплексонометрическое титрование;
по способу индикации конечной точки титровани€ Ц визуальное, потенциометрическое, фотометрическое, кондуктометрическое, амперометрическое титрование.
ѕо примен€емым реагентам титриметрические методы анализа подраздел€ютс€ на следующие виды:
Ц ацидометрическое титрование (титрант кислота Ц Ќ—1 или Ќ2SO4);
Ц алкалиметрическое титрование (титрант Ц щелочь Ц NaOH или Ba(OH)2);
Ц перманганатометрическое титрование (титрант Ц KћnO4);
Ц хроматометрическое титрование (титрант - K2Cr2O7);
Ц йодометрическое титрование (титрант I2 или KI) и т.д.
»сходное вещество Ц химическое соединение, используемое дл€ приготовлени€ раствора с точно известной концентрацией (первичный стандарт), удовлетвор€ющее р€ду требований: 1) вещество должно быть химически чистым; 2) состав вещества должен точно соответствовать формуле; 3) вещество должно быть устойчивым при хранении; 4) должно иметь возможно большую мол€рную массу эквивалента. Ћишь немногие вещества удовлетвор€ют этим требовани€м.
ѕервичный стандарт Ц раствор, концентраци€ которого точно известна, приготовленный из исходного вещества. ѕервичные стандарты используют как дл€ обычных титриметрических определений, так и дл€ установлени€ точной концентрации растворов вторичных стандартов.
‘иксанал Ц запа€нна€ ампула, в которой находитс€ определенное количество соответствующего вещества. ‘иксаналы используют дл€ приготовлени€ растворов первичных стандартов.
¬торичные ст андарты Ц растворы, приготовленные с примерно известной концентрацией, а затем их точную концентрацию устанавливают (стандартизируют) по раствору первичного стандарта.
“итрование провод€т двум€ способами: пипетировани€ и отдельных навесок.
ћетод пипетировани€ состоит в том, что навеску анализируемого вещества раствор€ют в мерной колбе, довод€т объем до метки и берут дл€ титровани€ определенные равные (аликвотные) объемы раствора пипеткой. ƒл€ вычислени€ используют формулу: C1V1 = C2V2
ћетод отдельных навесок Ц в конической колбе или стакане раствор€ют точную навеску в определенном количестве растворител€.
“итр Ц количество г. вещества содержащегос€ в 1 мл. раствора или эквивалентное определ€емому веществу. Ќапример, если титр H2SO4 равен 0,0049 г/мл, это значит, что каждый мл раствора содержит 0,0049 г. серной кислоты.
—тандартный раствор (титрант) Ц раствор с точно известной концентрацией.
ѕр€мое титрование Ц определ€емое вещество в процессе титровани€ непосредственно реагирует с раствором титранта:
ј + ¬ → продукты реакции, где ј Ц раствор определ€емого вещества; ¬ Ц раствор титранта.
ќбратное титрование Ц к раствору определ€емого вещества добавл€ют точно известное количество другого вещества в избытке (титрант 1); не вступившее в реакцию количество титранта 1 оттитровывают титрантом 2:
ј + ¬1 → продукты реакции + остаток ¬1,
¬1 + ¬2 → продукты реакции.
“итрование заместител€ (косвенное титрование) Ц к раствору определ€емого вещества добавл€ют вспомогательный раствор реагента (заведомо в избытке, дл€ смещени€ равновеси€ реакции вправо). ѕродукт реакции (заместитель), количество эквивалентов которого в точности равно количеству эквивалентов определ€емого вещества, оттитровывают раствором титранта:
ј + ƒ → продукты реакции + —,
— + ¬ → продукты реакции, где ƒ Ц раствор вспомогательного реагента; — Ц заместитель; ¬ Ц раствор
титранта.
Ёквивалент Ц реальна€ или условна€ частица вещества, котора€ в
кислотно-основной реакции эквивалентна одному иону водорода или (в
окислительно-восстановительной реакции) одному электрону.
‘актор эквивалентности [fэквив.(ј)] Ц число, обозначающее, кака€ дол€ реальной частицы вещества ј эквивалентна одному иону водорода или одному электрону.
|
|
|
ћол€рна€ масса эквивалента [ћ(ј)⋅fэквив.(ј)] Ц масса мол€ эквивалента, численно равна€ произведению фактора эквивалентности на мол€рную массу вещества.
ѕравило эквивалентов Ц вещества реагируют в объемах, обратно пропорциональных мол€рным концентраци€м их эквивалентов:
C1 ⋅ V1 = C2 ⋅V2.
ћол€рна€ концентраци€ эквивалента [—(ј)⋅f эквив. ] Ц число молей эквивалентов в 1 л раствора, моль/л.
“очка эквивалентности (т. эк.) Ц момент, когда определ€емое вещество полностью прореагировало с раствором титранта. “. эк. Ц пон€тие теоретическое.
“очка конца титровани€ (т. к. т.) Ц момент изменени€ физического свойства (изменение окраски) титруемого раствора, св€занный с эквивалентностью. „аще всего это изменение фиксируетс€ индикаторным или инструментальным способом. —ледовательно, т. к. т. Ц пон€тие практическое. –азность объемов титранта в т. эк. и т. к. т. мала, но она существует из-за неадекватности изменени€ физического свойства и нашей способности наблюдать его. V (т. эк.) ≠ V(т. к. т.). Ётим обусловливаетс€ наличие индикаторной ошибки титровани€.
»ндикаторы Ц химические вещества, измен€ющие окраску, люминесценцию или образующие осадок при изменении концентрации того или иного компонента в растворе. —уществуют кислотно-основные, окислительно-восстановительные, адсорбционные и комплексонометрические (металл индикаторы) индикаторы.
ѕ–»ћ≈–џ –≈Ў≈Ќ»я «јƒј„
ѕример 1. ќпределите массовую долю (ω, %) FeSO4Х7H2O в препарате одним из титриметрических методов анализа, если по предварительным данным она составл€ет около 90%.
–ешение. ƒл€ количественного определени€ можно использовать перманганатометрический метод анализа.
1) ќбъ€сните сущность определени€, докажите, что данное вещество можно определить предлагаемым методом: определение состоит в окислении ионов Fe2+ кали€ перманганатом в кислой среде, при этом расод титранта эквивалентен количеству определ€емого вещества.
2) Ќапишите уравненение реакции, обоснуйте величину мол€рной массы эквивалента определ€емого вещества (найдите в спавочнике Ћурье ё.ё.)

3) ќбоснуйте выбор способа фиксировани€ конечной точки титровани€: титрант окрашен (безындикаторное титрование), конечную точку титровани€ определ€ют по по€влению розового окрашивани€ от одной избыточной капли титранта.
4) –ассчитайте массу навески анализируемого вещества, если известно его приблизительное содержание. Ёто можно сделать либо способом отдельных навесок, либо способом пипетировани€. ¬ыбираем метод отдельных навесок. “огда
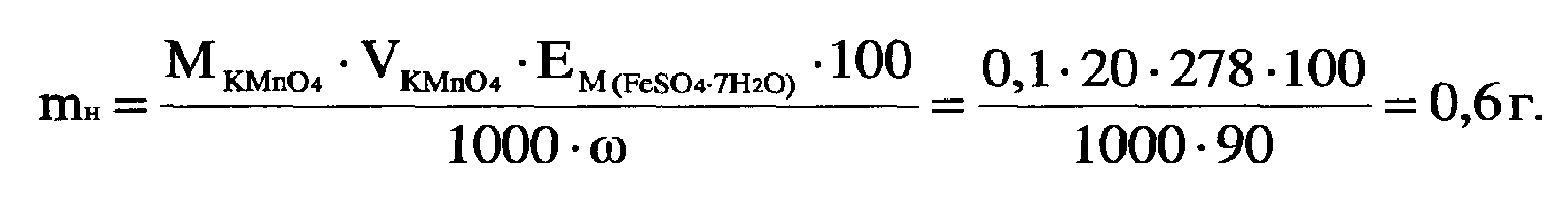
–ассчитанную навеску взвешивают на аналитических весах, перенос€т в пробирку с пробкой. Ќапример, mн = 0,5988 г.
5) ќпишите методику и услови€ определени€: вз€тую на аналитических весах навеску перенос€т в коническую колбу, раствор€ют в удобном дл€ титровани€ объеме воды (20-25 см3) и добавл€ют 20см3 2ћ H2SO4 и титруют 0,1ћ раствором KMnO4 с точно известной концентрацией до по€влени€ розовой окраски.
6) —оставьте и объ€сните решение задачи. –асчет проведите 2 способами:
а) по величине мол€рной массы эквивалента вещества;
б) по величине титра рабочего раствора по определ€емому веществу.

«јƒј„»
141. ќпределите массовую долю щавелевой кислоты одним из титриметрических методов анализа по схеме примера 1 (ω = 75%)
142. ќпределите массовую долю As2O3 одним из титриметрических методов анализа по схеме примера 1 (ω = 90%)
143. ќпределите массовую долю фенола одним из титриметрических методов анализа по схеме примера 1 (ω = 40%)
144. ќпределите массовую долю уксусной кислоты кислоты одним из титриметрических методов анализа по схеме примера 1 (ω = 80%)
145. ќпределите массовую долю CaCl2 одним из титриметрических методов анализа по схеме примера 1 (ω = 75%)
146. ќпределите массовую долю аммони€ бромида одним из титриметрических методов анализа по схеме примера 1 (ω = 80%)
147. ќпределите массовую долю аммони€ хлорида одним из титриметрических методов анализа по схеме примера 1 (ω = 80%)
148. ќпределите массовую долю железа (II) сульфата одним из титриметрических методов анализа по схеме примера 1 (ω = 60%)
149. ќпределите массовую долю железа (II) хлорида одним из титриметрических методов анализа по схеме примера 1 (ω = 70%)
150. ќпределите массовую долю натри€ хлорида одним из титриметрических методов анализа по схеме примера 1 (ω = 50%)
151. ќпределите массовую долю щавелевой кислоты одним из титриметрических методов анализа по схеме примера 1 (ω = 75%)
|
|
|
152. ќпределите массовую долю As2O3 одним из титриметрических методов анализа по схеме примера 1 (ω = 90%)
153. ќпределите массовую долю фенола одним из титриметрических методов анализа по схеме примера 1 (ω = 40%)
154. ќпределите массовую долю уксусной кислоты кислоты одним из титриметрических методов анализа по схеме примера 1 (ω = 80%)
155. ќпределите массовую долю CaCl2 одним из титриметрических методов анализа по схеме примера 1 (ω = 75%)
156. ќпределите массовую долю аммони€ бромида одним из титриметрических методов анализа по схеме примера 1 (ω = 80%)
157. ќпределите массовую долю аммони€ хлорида одним из титриметрических методов анализа по схеме примера 1 (ω = 80%)
158. ќпределите массовую долю железа (II) сульфата одним из титриметрических методов анализа по схеме примера 1 (ω = 60%)
159. ќпределите массовую долю железа (III) сульфата одним из титриметрических методов анализа по схеме примера 1 (ω = 70%)
160. ќпределите массовую долю (NH4)2—2ќ4 одним из титриметрических методов анализа по схеме примера 1 (ω = 50%)
¬ј–»јЌ“џ
| номер варианта | номера задач |
| 01, 21, 41, 61, 81 | 1, 21, 41, 61, 81, 101, 121, 141 |
| 02, 22, 42, 62, 82 | 2, 22, 42, 62, 82, 102, 122, 142 |
| 03, 23, 43, 63, 83 | 3, 23, 43, 63, 83, 103, 123, 143 |
| 04, 24, 44, 64, 84 | 4, 24, 44, 64, 84, 104, 124, 144 |
| 05, 25, 45, 65, 85 | 5, 25, 45, 65, 85, 105, 125, 145 |
| 06, 26, 46, 66, 86 | 6, 26, 46, 66, 86, 106, 126, 146 |
| 07, 27, 47, 67, 87 | 7, 27, 47, 67, 87, 107, 127, 147 |
| 08, 28, 48, 68, 88 | 8, 28, 48, 68, 88, 108, 128, 148 |
| 09, 29, 49, 69, 89 | 9, 29, 49, 69, 89, 109, 129, 149 |
| 10, 30, 50, 70, 90 | 10, 30, 50, 70, 90, 110, 130, 150 |
| 11, 31, 51, 71, 91 | 11, 31, 51,71, 91, 111, 131, 151 |
| 12, 32, 52, 72, 92 | 12, 32, 52, 72, 92, 112, 132, 152 |
| 13, 33, 53, 73, 93 | 13, 33, 53, 73, 93, 113, 133, 153 |
| 14, 34, 54, 74, 94 | 14, 34, 54, 74, 94, 114, 134, 154 |
| 15, 35, 55, 75, 95 | 15, 35, 55, 75, 95, 115, 135, 155 |
| 16, 36, 56, 76, 96 | 16, 36, 56, 76, 96, 116, 136, 156 |
| 17, 37, 57, 77, 97 | 17, 37, 57, 77, 97, 117, 137, 157 |
| 18, 38, 58, 78, 98 | 18, 38, 58, 78, 98, 118, 138, 158 |
| 19, 39, 59, 79, 99 | 19, 39, 59, 79, 99, 119, 139, 159 |
| 20, 40, 60, 80, 00 | 20, 40, 60, 80, 100, 120, 140, 160 |
—ѕ»—ќ –≈ ќћ≈Ќƒ”≈ћќ… Ћ»“≈–ј“”–џ
1. Ћогинов Ќ.я., ¬оскресенский ј.√. јналитическа€ хими€. ћ.: ѕросвещение, 1976.
2. јлексеев ¬.Ќ. урс качественного химического полумикроанализа. ћ.: ’ими€, 1973.
3. Ћурье ё.ё. —правочник по аналитической химии. ћ.: ’ими€, 1989.
4. лещев Ќ.‘., јлферова ≈.ј. «адачник по аналитической химии. ћ.: ’ими€, 1993.
5. ƒорохова ≈.Ќ. «адачи и вопросы по аналитической химии. ћ.: ћ√”, 1984.
6. ¬асильев ¬.ѕ. —борник вопросов и задач по аналитической химии. ћ.: ¬ысша€ школа, 1976.
7. ярославцев ј.ј. —борник задач и упражнений по аналитической химии. ћ.: ¬ысша€ школа, 1979.
ѕриложение.
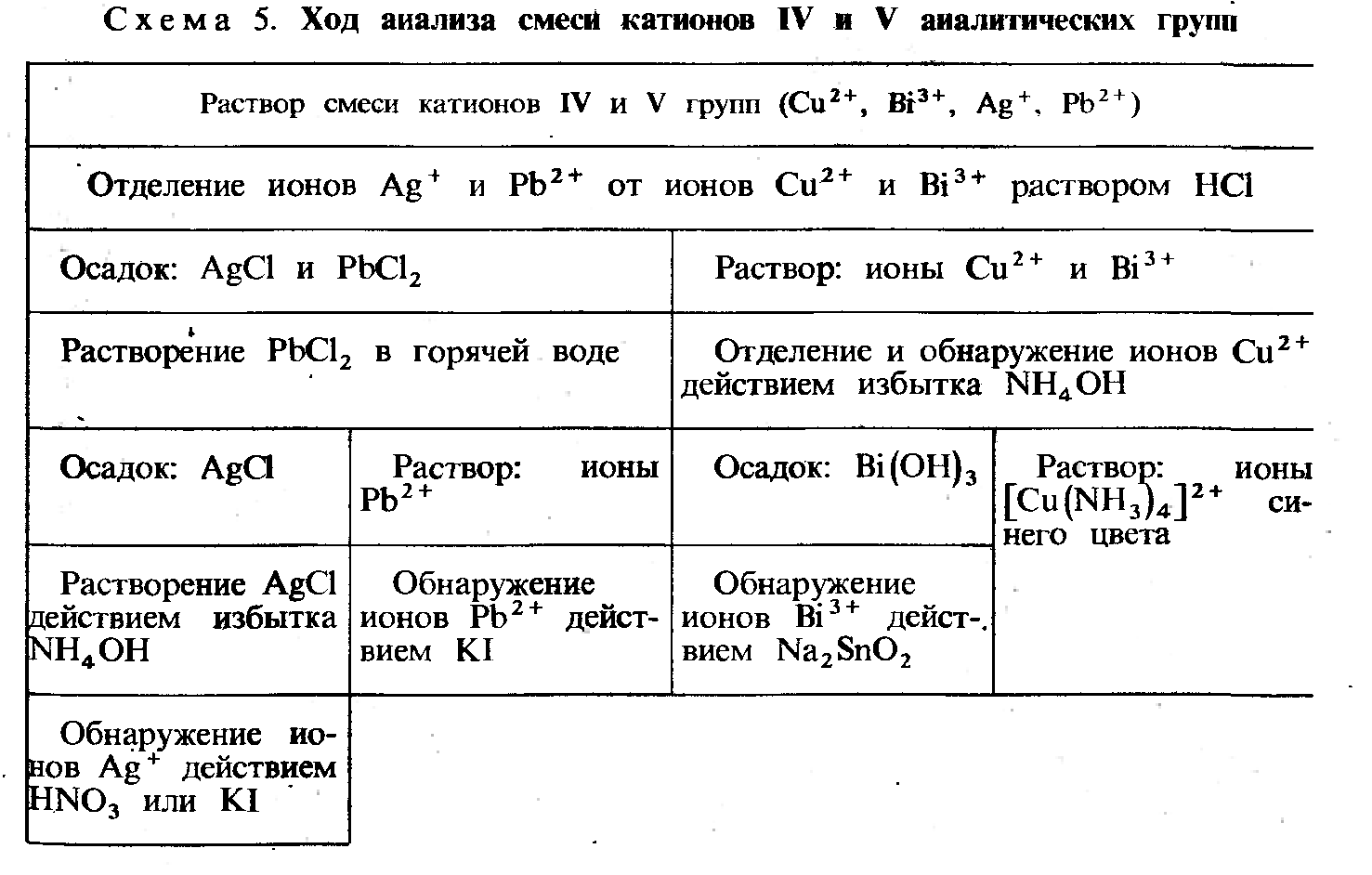
јналитическа€ кислотно-основна€ классификаци€ катионов.
| Ќомер группы | »оны | ’арактеристика группы | √рупповой реагент | ’арактер получаемых соединений |
| I | K+, Na+, NH4+ | ’лориды, сульфаты, основани€ растворимы в воде. | Ќе имеет | –аствор K+, Na+, NH4+ |
| II | Ag+, Pb2+ | ’лориды не растворимы в разбавленных кислотах. | 2 ћ раствор HCl | ќсадок белый AgCl PbCl2 |
| III | Ca2+, Ba2+, (Pb2+) | —ульфаты не растворимы в кислотах. | 1 ћ раствор H2SO4 | ќсадок белый CaSO4, BaSO4, (PbSO4) |
| IV | Al3+, Cr3+, Zn2+, Sn2+, (Sb3+) | ќсновани€ амфотерные и растворимы в избытке щелочи. | »збыток 4 ћ раствора щелочи (KOH, NaOH) | –астворы [Al(OH)4]- [Cr(OH)4]- [Zn(OH)4]2- [Sn(OH)3]- ([Sb(OH)4]-) |
| V | Mg2+, Mn2+, Fe2+, Fe3+, Sb3+ | ќсновани€ не растворимы в избытке щелочи. | »збыток 25% раствора аммиака (NH3H2O) | ќсадок Mg(OH)2 Mn(OH)2 Fe(OH)2 Fe(OH)3 Sb(OH)3,(H3SbO3) |
| VI | Cu2+, Co2+, Ni2+ | –астворимые комплексные аммиакаты | »збыток 25% раствора аммиака NH3H2O | –аствор [Cu(NH3)4]2+ [Co(NH3)6]2+ [Ni(NH3)6]2+ |
јналитическа€ классификаци€ анионов
| √руппа | јнионы | √рупповой реагент | ѕолучаемые соединени€ |
| I | SO42-, CO32-, PO43-,SiO32- | –аствор BaCl2 | ќсадки белого цвета BaSO4, BaCO3, Ba3(PO4)2, BaSiO3 |
| II | Cl-, S2- | –аствор AgNO3 в присутствии HNO3 | ќсадки AgCl, Ag2S белый черный |
| III | NO3- | - | - |
| IV | MoO42-, WO42-, VO3- | ћеталл Zn, HCl концентрированна€ | –аствор синий, зеленый, фиолетовый W2O5, Mo2O5, VOCl2 |