ќ —»ƒџ
ќксиды - это сложные вещества, состо€щие из двух химических элементов, один из которых кислород, с валентность равной 2. Ћишь один химический элемент - фтор, соедин€€сь с кислородом, образует не оксид, а фторид кислорода OF2.
Ќазываютс€ они просто - "оксид + название элемента" (см. таблицу). ≈сли валентность химического элемента переменна€, то указываетс€ римской цифрой, заключЄнной в круглые скобки, после названи€ химического элемента.
| ‘ормула | Ќазвание | ‘ормула | Ќазвание |
| CO | оксид углерода (II) | Fe2O3 | оксид железа (III) |
| NO | оксид азота (II) | CrO3 | оксид хрома (VI) |
| Al2O3 | оксид алюмини€ | ZnO | оксид цинка |
| N2O5 | оксид азота (V) | Mn2O7 | оксид марганца (VII) |
лассификаци€ оксидов
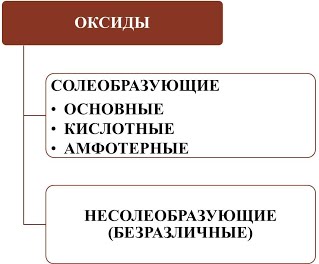
¬се оксиды можно разделить на две группы: солеобразующие (основные, кислотные, амфотерные) и несолеобразующие или безразличные.
| ќксиды металлов ћехќу | ќксиды неметалловнећехќу | |||
| ќсновные | ислотные | јмфотерные | ислотные | Ѕезразличные |
| I, II ће | V-VII Me | ZnO,BeO,Al2O3, Fe2O3, Cr2O3 | > II неће | I, II неће CO, NO, N2O |
1). ќсновные оксиды Ц это оксиды, которым соответствуют основани€. основным оксидам относ€тс€ оксидыметаллов 1 и 2 групп, а также металлов побочных подгрупп с валентностью I и II (кроме ZnO - оксид цинка и BeO Ц оксид берили€):

2). ислотные оксиды Ц это оксиды, которым соответствуют кислоты. кислотным оксидам относ€тс€ оксиды неметаллов (кроме несолеобразующих Ц безразличных), а также оксиды металлов побочных подгрупп с валентностью от V до VII (Ќапример, CrO3-оксид хрома (VI), Mn 2O7 - оксид марганца (VII)):

3). јмфотерные оксиды Ц это оксиды, которым соответствуют основани€ и кислоты. ним относ€тс€ оксиды металлов главных и побочных подгрупп с валентностью III, иногда IV, а также цинк и бериллий (Ќапример, BeO, ZnO, Al2O3, Cr2O3).
4). Ќесолеобразующие оксиды Ц это оксиды безразличные к кислотам и основани€м. ним относ€тс€ оксиды неметаллов с валентностью I и II (Ќапример,N2O, NO, CO).
¬ывод: характер свойств оксидов в первую очередь зависит от валентности элемента.
Ќапример, оксиды хрома:
CrO (II - основный);
Cr 2O3 (III - амфотерный);
CrO3 (VII - кислотный).
’имические свойства оксидов
| ’»ћ»„≈— »≈ —¬ќ…—“¬ј ќ—Ќќ¬Ќџ’ ќ —»ƒќ¬ 1. ќсновной оксид + ислотный оксид = —оль (р. соединени€) CaO + SO2 = CaSO3 2. ќсновной оксид + ислота = —оль + Ќ2ќ (р. обмена) 3K2O + 2H3PO4 = 2K3PO4 + 3H2O 3. ќсновной оксид + ¬ода = ўЄлочь (р. соединени€) Na2O + H2O = 2NaOH |
| ’»ћ»„≈— »≈ —¬ќ…—“¬ј »—Ћќ“Ќџ’ ќ —»ƒќ¬ 1. ислотный оксид + ¬ода = ислота (р. соединени€) —O2 + H2O = H2CO3, SiO2 Ц не реагирует 2. ислотный оксид + ќснование = —оль + Ќ2ќ (р. обмена) P2O5 + 6KOH = 2K3PO4 + 3H2O 3. ќсновной оксид + ислотный оксид = —оль (р. соединени€) CaO + SO2 = CaSO3 4. ћенее летучие вытесн€ют более летучие из их солей CaCO3 + SiO2 = CaSiO3 +CO2 |
| ’»ћ»„≈— »≈ —¬ќ…—“¬ј јћ‘ќ“≈–Ќџ’ ќ —»ƒќ¬ ¬заимодействуют как с кислотами, так и со щелочами. ZnO + 2 HCl = ZnCl2 + H2O ZnO + 2 NaOH + H2O = Na2[Zn(OH)4] (в растворе) ZnO + 2 NaOH = Na2ZnO2 + H2O (при сплавлении) |
|
|
|
ѕрименение оксидов
Ќекоторые оксиды не раствор€ютс€ в воде, но многие вступают с водой в реакции соединени€:
SO3 + H2O = H2SO4
CaO + H2O = Ca(OH)2
¬ результате часто получаютс€ очень нужные и полезные соединени€. Ќапример, H2SO4 Ц серна€ кислота, —а(ќЌ)2 Ц гашена€ известь и т.д.
≈сли оксиды нерастворимы в воде, то люди умело используют и это их свойство. Ќапример, оксид цинка ZnO Ц вещество белого цвета, поэтому используетс€ дл€ приготовлени€ белой масл€ной краски (цинковые белила). ѕоскольку ZnO практически не растворим в воде, то цинковыми белилами можно красить любые поверхности, в том числе и те, которые подвергаютс€ воздействию атмосферных осадков. Ќерастворимость и не€довитость позвол€ют использовать этот оксид при изготовлении косметических кремов, пудры. ‘армацевты делают из него в€жущий и подсушивающий порошок дл€ наружного применени€.
“акими же ценными свойствами обладает оксид титана (IV) Ц TiO2. ќн тоже имеет красивый белый цвет и примен€етс€ дл€ изготовлени€ титановых белил. TiO2 не раствор€етс€ не только в воде, но и в кислотах, поэтому покрыти€ из этого оксида особенно устойчивы. Ётот оксид добавл€ют в пластмассу дл€ придани€ ей белого цвета. ќн входит в состав эмалей дл€ металлической и керамической посуды.
ќксид хрома (III) Ц Cr2O3 Ц очень прочные кристаллы темно-зеленого цвета, не растворимые в воде. Cr2O3 используют как пигмент (краску) при изготовлении декоративного зеленого стекла и керамики. »звестна€ многим паста √ќ» (сокращение от наименовани€ У√осударственный оптический институтФ) примен€етс€ дл€ шлифовки и полировки оптики, металлических изделий, в ювелирном деле.
Ѕлагодар€ нерастворимости и прочности оксида хрома (III) его используют и в полиграфических красках (например, дл€ окраски денежных купюр). ¬ообще, оксиды многих металлов примен€ютс€ в качестве пигментов дл€ самых разнообразных красок, хот€ это Ц далеко не единственное их применение.
ќснóвные гидроксиды содержат гидроксогруппы ќЌ−, способные замещатьс€ на кислотные остатки. ѕримеры:
NaOH - гидроксид натри€, LiOH - гидроксид лити€, Ba(OH)2 - гидроксид бари€,
Cu(OH)2 - гидроксид меди(II), La(OH)3 - гидроксид лантана(III).
–еакци€ нейтрализации
¬ажнейшим химическим свойством основных и кислотных гидроксидов €вл€етс€ их взаимодействие их между собой с образованием солей (реакци€ нейтрализации, или солеобразовани€), например:
Ca(OH)2 + H2SO4 = CaSO4 + 2H2O
Ca(OH)2 + 2H2SO4 = Ca(HSO4)2 + 2H2O
2Ca(OH)2 + H2SO4 = Ca2SO4(OH)2 + 2H2O
√идрокси́ды (гидроо́киси, водокиси) Ч неорганические соединени€, содержащие в составе гидроксильную группу -OH. »звестны гидроксиды почти всех химических элементов; некоторые из них встречаютс€ в природе в виде минералов. √идроксиды щелочных и щЄлочноземельных металлов, талли€, а также аммони€ €вл€ютс€ растворимыми и их называют щелочами.
¬ зависимости от того, €вл€етс€ ли соответствующий оксид основным, кислотным или амфотерным, соответственно различают:
Ј основные гидроксиды (основани€) Ч только гидроксиды металлов со степенью окислени€ +1, +2, про€вл€ющие основные свойства (например, гидроксид кальци€ Ca(ќЌ)2, гидроксид кали€ KOH, гидроксид натри€ NaOH и др.) ѕри реакци€х и диссоциации отщепл€етс€ группа -OH.
|
|
|
Ј кислотные гидроксиды (кислородсодержащие кислоты) Ч гидроксиды неметаллов и металлов со степенью окислени€ +5, +6, про€вл€ющие кислотные свойства (например, азотна€ кислота HNO3, серна€ кислота H2SO4, серниста€ кислота H2SO3 и др.) ѕри реакци€х и диссоциации отщепл€етс€ протон.
Ј амфотерные гидроксиды, гидроксиды металлов со степенью окислени€ +3, +4 и нескольких металлов со степенью окислени€ +2, которые про€вл€ют амфотерные свойства. јмфотерные гидроксиды про€вл€ют в зависимости от условий либо основные, либо кислотные свойства (например, гидроксид алюмини€ Al(ќЌ)3, гидроксид цинка Zn(ќЌ)2).
“ермин Ђгидроксидыї часто примен€ют только по отношению к основным и амфотерным гидроксидам. “акже иногда называют гидроксидом воду.
’имические свойства[править | править вики-текст]
ќсновные гидроксиды[править | править вики-текст]
ќксиды щелочных и некоторых щëлочноземельных металлов взаимодействуют с водой, образу€ щëлочи:
{\displaystyle {\mathsf {Na_{2}O+H_{2}O\rightarrow 2NaOH}}} 
{\displaystyle {\mathsf {CaO+H_{2}O\rightarrow Ca(OH)_{2}}}} 
Ќерастворимые основани€ при нагревании, как правило, разлагаютс€ на оксид и воду, например:
{\displaystyle {\mathsf {2Fe(OH)_{3}\rightarrow Fe_{2}O_{3}+3H_{2}O}}} 
{\displaystyle {\mathsf {Cu(OH)_{2}\rightarrow CuO+H_{2}O}}} 
ислотные гидроксиды[править | править вики-текст]
ѕри взаимодействии оксида неметалла с водой образуетс€ кислородсодержаща€ кислота:
{\displaystyle {\mathsf {SO_{3}+H_{2}O\rightarrow H_{2}SO_{4}}}} 
15. ¬одород (лат. Hydrogenium), H, химический элемент, первый по пор€дковому номеру в периодической системе ћенделеева; атомна€ масса 1,0079. ѕри обычных услови€х ¬одород - газ; не имеет цвета, запаха и вкуса.
»сторическа€ справка. ¬ трудах химиков 16 и 17 веков неоднократно упоминалось о выделении горючего газа при действии кислот на металлы. ¬ 1766 году √. авендиш собрал и исследовал выдел€ющийс€ газ, назвав его "горючий воздух". Ѕудучи сторонником теории флогистона, авендиш полагал, что этот газ и есть чистый флогистон. ¬ 1783 году ј. Ћавуазье путем анализа и синтеза воды доказал сложность ее состава, а в 1787 определил "горючий воздух" как новый химический элемент (¬одород) и дал ему современное название hydrogene (от греч. hydor - вода и gennao - рождаю), что означает "рождающий воду"; этот корень употребл€етс€ в названи€х соединений ¬одорода и процессов с его участием (например, гидриды, гидрогенизаци€). —овременное русское наименование "¬одород" было предложено ћ. ‘. —оловьевым в 1824 году.
–аспространение ¬одорода в природе. ¬одород широко распространен в природе, его содержание в земной коре (литосфера и гидросфера) составл€ет по массе 1%, а по числу атомов 16%. ¬одород входит в состав самого распространенного вещества на «емле - воды (11,19% ¬одорода по массе), в состав соединений, слагающих угли, нефть, природные газы, глины, а также организмы животных и растений (то есть в состав белков, нуклеиновых кислот, жиров, углеводов и других). ¬ свободном состо€нии ¬одород встречаетс€ крайне редко, в небольших количествах он содержитс€ в вулканических и других природных газах. Ќичтожные количества свободного ¬одорода (0,0001% по числу атомов) присутствуют в атмосфере. ¬ околоземном пространстве ¬одород в виде потока протонов образует внутренний ("протонный") радиационный по€с «емли. ¬ космосе ¬одород €вл€етс€ самым распространенным элементом. ¬ виде плазмы он составл€ет около половины массы —олнца и большинства звезд, основную часть газов межзвездной среды и газовых туманностей. ¬одород присутствует в атмосфере р€да планет и в кометах в виде свободного Ќ2, метана —Ќ4, аммиака NH3, воды Ќ2ќ, радикалов типа CH, NH, OH, SiH, PH и т. д. ¬ виде потока протонов ¬одород входит в состав корпускул€рного излучени€ —олнца и космических лучей.
»зотопы, атом и молекула ¬одорода. ќбыкновенный ¬одород состоит из смеси 2 устойчивых изотопов: легкого ¬одорода, или проти€ (1H), и т€желого ¬одорода, или дейтери€ (2Ќ, или D). ¬ природных соединени€х ¬одорода на 1 атом 2Ќ приходитс€ в среднем 6800 атомов 1Ќ. –адиоактивный изотоп с массовым числом 3 называют сверхт€желым ¬одородом, или тритием (3Ќ, или “), с м€гким β-излучением и периодом полураспада T½ = 12,262 года. ¬ природе тритий образуетс€, например, из атмосферного азота под действием нейтронов космических лучей; в атмосфере его ничтожно мало (4Ј10-15% от общего числа атомов ¬одорода). ѕолучен крайне неустойчивый изотоп 4Ќ. ћассовые числа изотопов 1Ќ, 2Ќ, 3Ќ и 4Ќ, соответственно 1, 2, 3 и 4, указывают на то, что €дро атома проти€ содержит только один протон, дейтери€ - один протон и один нейтрон, трити€ - один протон и 2 нейтрона, 4Ќ - один протон и 3 нейтрона. Ѕольшое различие масс изотопов ¬одорода обусловливает более заметное различие их физических и химических свойств, чем в случае изотопов других элементов.
|
|
|
јтом ¬одорода имеет наиболее простое строение среди атомов всех других элементов: он состоит из €дра и одного электрона. Ёнерги€ св€зи электрона с €дром (потенциал ионизации) составл€ет 13,595 эв. Ќейтральный атом ¬одород может присоедин€ть и второй электрон, образу€ отрицательный ион Ќ- при этом энерги€ св€зи второго электрона с нейтральным атомом (сродство к электрону) составл€ет 0,78 эв. вантова€ механика позвол€ет рассчитать все возможные энергетические уровни атома ¬одород, а следовательно, дать полную интерпретацию его атомного спектра. јтом ¬одорода используетс€ как модельный в квантовомеханических расчетах энергетических уровней других, более сложных атомов.
ћолекула ¬одород Ќ2 состоит из двух атомов, соединенных ковалентной химической св€зью. Ёнерги€ диссоциации (то есть распада на атомы) составл€ет 4,776 эв. ћежатомное рассто€ние при равновесном положении €дер равно 0,7414Å. ѕри высоких температурах молекул€рный ¬одород диссоциирует на атомы (степень диссоциации при 2000∞— 0,0013, при 5000∞— 0,95). јтомарный ¬одород образуетс€ также в различных химических реакци€х (например, действием Zn на сол€ную кислоту). ќднако существование ¬одорода в атомарном состо€нии длитс€ лишь короткое врем€, атомы рекомбинируют в молекулы Ќ2.
‘изические свойства ¬одорода. ¬одород - легчайшее из всех известных веществ (в 14,4 раза легче воздуха), плотность 0,0899 г/л при 0∞— и 1 атм. ¬одород кипит (сжижаетс€) и плавитс€ (затвердевает) соответственно при -252,8∞— и -259,1∞— (только гелий имеет более низкие температуры плавлени€ и кипени€). ритическа€ температура ¬одорода очень низка (-240∞—), поэтому его сжижение сопр€жено с большими трудност€ми; критическое давление 12,8 кгс/см2 (12,8 атм), критическа€ плотность 0,0312 г/см3. »з всех газов ¬одород обладает наибольшей теплопроводностью, равной при 0∞— и 1 атм 0,174 вт/(мЈ ), то есть 4,16Ј10-4 кал/(сЈсмЈ∞—). ”дельна€ теплоемкость ¬одорода при 0∞— и 1 атм —p 14,208 кƒж/(кгЈ ), то есть 3,394 кал/(гЈ∞—). ¬одород мало растворим в воде (0,0182 мл/г при 20∞— и 1 атм), но хорошо - во многих металлах (Ni, Pt, Pa и других), особенно в палладии (850 объемов на 1 объем Pd). — растворимостью ¬одорода в металлах св€зана его способность диффундировать через них; диффузи€ через углеродистый сплав (например, сталь) иногда сопровождаетс€ разрушением сплава вследствие взаимодействи€ ¬одорода с углеродом (так называема€ декарбонизаци€). ∆идкий ¬одород очень легок (плотность при -253∞— 0,0708 г/см3) и текуч (в€зкость при -253∞— 13,8 спуаз).
’имические свойства ¬одорода. ¬ большинстве соединений ¬одород про€вл€ет валентность (точнее, степень окислени€) +1, подобно натрию и другим щелочным металлам; обычно он и рассматриваетс€ как аналог этих металлов, возглавл€ющий I группу системы ћенделеева. ќднако в гидридах металлов ион ¬одорода зар€жен отрицательно (степень окислени€ -1), то есть гидрид Na+H- построен подобно хлориду Na+Cl-. Ётот и некоторые других факты (близость физических свойств ¬одорода и галогенов, способность галогенов замещать ¬одород в органических соединени€х) дают основание относить ¬одород также и к VII группе периодической системы. ѕри обычных услови€х молекул€рный ¬одород сравнительно мало активен, непосредственно соедин€€сь лишь с наиболее активными из неметаллов (с фтором, а на свету и с хлором). ќднако при нагревании он вступает в реакции со многими элементами. јтомарный ¬одород обладает повышенной химические активностью по сравнению с молекул€рным. — кислородом ¬одород образует воду:
|
|
|
Ќ2 + 1/2ќ2 = Ќ2ќ
с выделением 285,937 кƒж/моль, то есть 68,3174 ккал/моль тепла (при 25∞— и 1 атм). ѕри обычных температурах реакци€ протекает крайне медленно, выше 550∞— - со взрывом. ѕределы взрывоопасности водородо-кислородной смеси составл€ют (по объему) от 4 до 94% Ќ2, а водородо-воздушной смеси - от 4 до 74% Ќ2 (смесь 2 объемов Ќ2 и 1 объема ќ2называетс€ гремучим газом). ¬одород используетс€ дл€ восстановлени€ многих металлов, так как отнимает кислород у их оксидов:
CuO + H2 = Cu + H2O,
Fe3O4 + 4H2 = 3Fe + 4Ќ2ќ, и т. д.
— галогенами ¬одород образует галогеноводороды, например:
Ќ2 + Cl2 = 2Ќ—l.
ѕри этом с фтором ¬одород взрываетс€ (даже в темноте и при - 252∞—), с хлором и бромом реагирует лишь при освещении или нагревании, а с иодом только при нагревании. — азотом ¬одород взаимодействует с образованием аммиака:
«Ќ2 + N2 = 2NЌ3
лишь на катализаторе и при повышенных температуpax и давлени€х. ѕри нагревании ¬одород энергично реагирует с серой:
Ќ2 + S = H2S (сероводород),
значительно труднее с селеном и теллуром. — чистым углеродом ¬одород может реагировать без катализатора только при высоких температуpax:
2Ќ2 + — (аморфный) = —Ќ4 (метан).
¬одород непосредственно реагирует с некоторыми металлами (щелочными, щелочноземельными и другими), образу€ гидриды:
Ќ2 + 2Li = 2LiH.
¬ажное практическое значение имеют реакции ¬одорода с оксидом углерода (II), при которых образуютс€ в зависимости от температуры, давлени€ и катализатора различные органические соединени€, например Ќ—Ќќ, —Ќ3ќЌ и другие. Ќенасыщенные углеводороды реагируют с ¬одородом, переход€ в насыщенные, например:
—nЌ2n + Ќ2 = —nЌ2n+2.
–оль ¬одород и его соединений в химии исключительно велика. ¬одород обусловливает кислотные свойства так называемых протонных кислот. ¬одород склонен образовывать с некоторыми элементами так называемую водородную св€зь, оказывающую определ€ющее вли€ние на свойства многих органических и неорганических соединений.
ѕолучение ¬одорода. ќсновные виды сырь€ дл€ промышленного получени€ ¬одорода - газы природные горючие, коксовый газ и газы нефтепереработки. ¬одород получают также из воды электролизом (в местах с дешевой электроэнергией). ¬ажнейшими способами производства ¬одорода из природного газа €вл€ютс€ каталитическое взаимодействие углеводородов, главным образом метана, с вод€ным паром (конверси€):
—Ќ4 + H2ќ = —ќ + «Ќ2,
и неполное окисление углеводородов кислородом:
—Ќ4 + 1/2ќ2 = —ќ + 2Ќ2
ќбразующийс€ оксид углерода (II) также подвергаетс€ конверсии:
—ќ + Ќ2ќ = —ќ2 + Ќ2.
¬одород, добываемый из природного газа, самый дешевый.
»з коксового газа и газов нефтепереработки ¬одород выдел€ют путем удалени€ остальных компонентов газовой смеси, сжижаемых более легко, чем ¬одород, при глубоком охлаждении. Ёлектролиз воды ведут посто€нным током, пропуска€ его через раствор ќЌ или NaOH (кислоты не используютс€ во избежание коррозии стальной аппаратуры). ¬ лаборатори€х ¬одород получают электролизом воды, а также по реакции между цинком и сол€ной кислотой. ќднако чаще используют готовый заводской ¬одород в баллонах.
ѕрименение ¬одорода. ¬ промышленном масштабе ¬одород стали получать в конце 18 века дл€ наполнени€ воздушных шаров. ¬ насто€щее врем€ ¬одород широко примен€ют в химической промышленности, главным образом дл€ производства аммиака. рупным потребителем ¬одорода €вл€етс€ также производство метилового и других спиртов, синтетического бензина и других продуктов, получаемых синтезом из ¬одорода и оксида углерода (II). ¬одород примен€ют дл€ гидрогенизации твердого и т€желого жидкого топлив, жиров и других, дл€ синтеза HCl, дл€ гидроочистки нефтепродуктов, в сварке и резке металлов кислородо-водородным пламенем (температура до 2800∞—) и в атомно-водородной сварке (до 4000∞—). ќчень важное применение в атомной энергетике нашли изотопы ¬одорода - дейтерий и тритий.
|
|
|






