ѕример 1. ѕри сгорании 1 кг метана выделилось 50137,5 кƒж теплоты. –ассчитайте стандартную энтальпию образовани€ метана ∆Ќ о—Ќ4.
–ешение.
1. ѕересчитаем количество участвующего в реакции метана, выраженное в граммах, в моли (учитыва€, что мол€рна€ масса —Ќ4 равна 16 г/моль):
оличество —Ќ4в мол€х n равно:
n —Ќ4=  =62,5 моль.
=62,5 моль.
2. –ассчитаем количество теплоты, выдел€ющеес€ при сгорании 1 мол€ метана:

3. «апишем термохимическое уравнение реакции горени€ метана:
CH4(г) + 2ќ2(г) = Cќ2(г) + 2Ќ2ќ(г), Q р=802,2 кƒж,
Q р=− ∆Ќ ор=(∆Ќ о—ќ2(г) +2 ∆Ќ оЌ2ќ(г))−(∆Ќ о—Ќ4(г) +2 ∆Ќ оќ2(г))=
=[(−393,5)+2(−241,8)−(∆Ќ о—Ќ4(г))−2(0)]=−802,2 кƒж.
ќтсюда: ∆Ќ о—Ќ4=(802,2−393,5−483,6)=−74,9 кƒж/моль —Ќ4.
ѕример 2. –ассчитайте количество теплоты, которое выделитс€ при полном сгорании 100 л этана, вз€того в газообразном состо€нии при н.у., если в результате реакции образуетс€ —ќ2(г) и Ќ2ќ(г).
–ешение.
1. –еакци€ горени€ этана выражаетс€ термохимичес≠ким уравнением
—2Ќ6(г) + «,5O2(г) = 2CO2(г) + 3Ќ2ќ(г); ∆Ќ р=−1559,87 кƒж.
2. ѕереведем количество участвующего в реакции этана, выраженное в литрах (н.у.), в моли (учитыва€, что 1 моль газа при н.у. занимает объем, равный 22,4 л):
оличество этана в мол€х n равно:
n —2Ќ6(г)=  =4,46 моль.
=4,46 моль.
3. Ќаходим значени€ стандартных энтальпий образовани€ (∆Ќ о298) дл€ всех веществ, участвующих в реакции (ѕрил. табл. 2) и рассчитываем тепловой эффект в расчете на один моль —2Ќ6(г):
∆Ќ ореакции=(2 ∆Ќ о—O2(г) +3 ∆Ќ оЌ2O (г))−(∆Ќ о—2Ќ6(г) +3,5 ∆Ќ оO2(г))=
= [2(−393,5)+3(−241,8)]−[−84,7+3,5×0]=−1427,7 кƒж/моль —2Ќ6(г).
Q р=− ∆Ќ ор=1427,7 кƒж.
4. ѕересчитаем полученный тепловой эффект на реальное количество этана, т.е. на 4,46 мол€ (100 л, н.у.):
Q р=− ∆Ќ ореальн.=1427,7×4,46=5767,42 кƒж.
ѕример 3. Ќе производ€ вычислений, определите знак изменени€ энтропии в следующих реакци€х. –ассчитайте изменение энтропии дл€ стандартных условий и сравните с результатом оценки.
1) 2NЌ3(г) = N2(г) + 3H2(г),
2) NH4NO3(тв) = N2O(г) + 2Ќ2ќ(г),
3) 2Ќ2(г) + O2(г) = 2Ќ2O(г),
4) 2Ќ2(г) + O2(г) = 2Ќ2O(ж).
–ешение. »зменение энтропии реакции можно оценить качественно в случае реакции с участием газов. ѕри переходе вещества в газообразное состо€ние происходит сильное увеличение энтропии, превышающее другие факторы, вли€ющие на энтропию. ѕоэтому по количеству вещества газов в правой и левой част€х уравнени€ реакции можно определить, возрастает энтропи€ или уменьшаетс€.
¬ первой реакции из 2-х молей вещества, наход€щегос€ в газообразном состо€нии образуетс€ 4 мол€ веществ, наход€щихс€ в газообразном состо€нии, следовательно, S 1>0.
»зменение энтропии этой реакции в стандартных услови€х (∆S о298) равно:
∆S о298= S оN2(г)+3 S оЌ2(г)−2 S оNЌ3(г)=191,5+ 3×130,5−2×192,7=197,6 ƒж/ .
|
|
|
¬о второй реакции 1 моль вещества в твердом состо€нии образует 3 мол€ газообразных веществ, следовательно, ∆S 2>0. »зменение энтропии этой реакции в стандартных услови€х (∆S о298) равно:
∆S о298= S оN2ќ(г)+2 S оЌ2ќ(г) Ц S оNЌ4NO3(тв) =219,8+2×188,7−151=446,2 ƒж/ .
¬ (3) и (4) реакци€х уменьшаетс€ как общее число молей, так и число молей газообразных веществ, так что ∆S 3<0 и ∆S 4<0, при этом D S 4 имеет более отрицательное значение, т.е. больше по абсолютной величине чем ∆S 3, так как S Ќ2ќ(г) > S Ќ2ќ(ж).
ѕример 4. ”становите возможность восстановлени€ диоксида титана до свободного металла по следующей реакции при стандартных услови€х и при 2500 (зависимостью ∆H ори ∆S орот температуры пренебречь):
TiO2(тв) + 2—(тв) = Ti(тв) + 2—ќ(г).
–ешение. »з второго закона термодинамики следует, что самопроизвольно протекают только такие реакции, которые сопровождаютс€ уменьшением энергии √иббса (∆G р<0).
1. –ассчитаем ∆ G ор дл€ стандартных условий с учетом табличных данных (см. прил. табл. 2):
∆ G орекции=(2∆ G о—ќ(г)+∆ G оTi(тв))−(∆ G оTiO2(тв) +2 ∆G о—(тв)) =
= [2(−137,1)+0]−[(−888,6)−2×0] =614,4 кƒж.
ѕоскольку ∆ G ор>0, реакци€ при 298 невозможна.
2. –ассчитаем изменение энергии √иббса этой реакции дл€ 2500 , дл€ чего воспользуемс€ уравнением:
∆G т=∆ Hо p− T∆S оp.
Ќаходим изменени€ ∆H оpи ∆S оpпри стандартных услови€х:
∆H оpеакции=(2 ∆H о—ќ(г) + ∆H о Ti(тв))−(∆H о TiO2(тв)+2 ∆H о —(тв)) =
= [2(−110,5)+0]−[(−943,9)−2×0 ]=722,9 кƒж=722900 ƒж.
∆S оpеакции=(2 S о—ќ(г)+ S оTi(тв))−(S оTiO2(тв)+ S о—(тв))=
= [2×197,5+30,6]−[50,3-2×5,7 ]=363,9 ƒж/ .
∆ Gт= ∆H ор− T∆ Sор=722900−2500×363,9=−186850 ƒж.
∆G 2500=−186,85 кƒж.
ѕоскольку ∆G 2500< 0, то реакци€ при 2500 возможна.
ѕример 5. ¬ычислите температуру, при которой в стандартном состо€нии установитс€ равновесии реакции:
2 NO2(г) 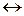 N2O4(г),
N2O4(г),
если известны: ∆Ќ ореакции=−55,3 кƒж; ∆S ореакции=−175,8 ƒж/ .
–ешение. »спользу€ дл€ расчета стандартные значени€ функций состо€ни€, имеем в виду стандартное состо€ние системы. ≈сли одновременно это равновесное состо€ние, то ∆G ореакции=0. ѕримен€ем уравнение:
∆G ор= ∆H ор− T∆ Sор=0.
ѕреобразуем его и подставл€ем числовые значени€:
 =314,56 (41,4о—).
=314,56 (41,4о—).
—истема находитс€ в равновесном стандартном состо€нии при температуре 41,4 о—.






