–астворы электролитов
ѕримеры решени€ задач
«адача 1. ¬ычислить значение рЌ водного раствора хлорноватистой кислоты HClO с мол€рной концентрацией 0,005 моль/л, содержащего также гипохлорид натри€ NaClO в концентрации 10−3 моль/л (степень диссоциации соли составл€ет 90%).
–ешение. —огласно значению константы кислотности хлорноватистой кислоты K а = 2,8∙10−8, HClO €вл€етс€ слабым электролитом и диссоциирует по уравнению
HClO ⇄ H+ + ClO−.
¬ присутствии соли NaClO положение равновеси€ диссоциации кислоты, в соответствии с принципом Ће Ўателье, сместитс€ в сторону образовани€ HClO в результате по€влени€ в растворе гипохлорид-анионов ClO− за счет диссоциации сильного электролита:
NaClO → Na+ + ClO−.
ѕри этом процесс диссоциации слабой кислоты будет подавлен, равновесна€ концентраци€ ионов водорода уменьшитс€ и составит x моль/л. “ак как ClO− образуютс€ вследствие диссоциации обоих электролитов, то их обща€ концентраци€ в растворе составл€ет
 = x + α NaClO ∙ C NaClO = (x + 0,9∙10−3) моль/л.
= x + α NaClO ∙ C NaClO = (x + 0,9∙10−3) моль/л.
онцентраци€ же недиссоциированной кислоты составит (0,005 Ц x) моль/л.
ѕодставим равновесные концентрации H+, ClO− и HClO в выражение константы диссоциации хлорноватистой кислоты и рассчитаем значение концентрации ионов водорода
= 2,8∙10−8
 = 1,55∙10−8 моль/л.
= 1,55∙10−8 моль/л.
ƒл€ слабых электролитов величину водородного показател€ раствора можно вычислить по формуле рЌ = −lg= −lg(1,55∙10−8) = 7,8.
“аким образом, среда в растворе кислоты с добавлением ее соли €вл€етс€ не слабокислотной, а слабощелочной, что обусловлено, помимо подавлени€ диссоциации HClO, еще и гидролизом соли NaClO по аниону.
«адача 2. –ассчитать значение рЌ раствора, полученного смешением 100 мл сантимол€рного раствора азотной кислоты HNO3 и 200 мл миллимол€рного раствора гидроксида бари€ Ba(OH)2.
–ешение. ѕри смешивании водных растворов азотной кислоты и гидроксида бари€ происходит реакци€ нейтрализации
2HNO3 + Ba(OH)2 → Ba(NO3)2 + 2H2O
¬ результате нее образуетс€ соль нитрат бари€ Ba(NO3)2, анион и катион которой обладают слабым пол€ризующим действием на молекулы воды. ѕоэтому данна€ соль в водных растворах практически не гидролизована, и рЌ раствора, полученного после реакции нейтрализации, будет определ€тьс€ тем исходным электролитом, который вз€т в избытке.
–ассчитаем число моль эквивалентов HNO3 и Ba(OH)2, содержащихс€ в исходных растворах по формуле
n э = — э Vz,
где — э Цмол€рна€ концентраци€ эквивалента (моль/л), V Ц объем раствора (л), z Ц число эквивалентности
n э (HNO3) = 10−2∙100∙10−3∙1 = 10−3 моль
n э (Ba(OH)2) = 10−3∙200∙10−3∙2 = 4∙10−4 моль
—огласно закону эквивалентов, азотна€ кислота дана в избытке, и по окончании реакции нейтрализации раствор будет содержать 6∙10−4 моль эквивалентов HNO3 и 4∙10−4 моль эквивалентов Ba(NO3)2.
|
|
|
¬ычислим мол€рные концентрации ионов в полученном после смешени€ электролитов растворе. “ак как азотна€ кислота и нитрат бари€ в водных растворах €вл€ютс€ сильными электролитами, то в соответствии с уравнением диссоциации
HNO3 → H+ + NO3−
Ba(NO3)2 → Ba2+ + 2NO3−,
концентрации ионов можно рассчитать на основании концентрации этих электролитов по формуле
,
что составл€ет
C (H+) =  = 2∙10−3 моль/л
= 2∙10−3 моль/л
C (Ba2+) =  = 6,67∙10−4 моль/л
= 6,67∙10−4 моль/л
C (NO3−) = C (H+) + 2 C (Ba2+) = 2∙10−3 + 2∙6,67∙10−4 = 3,33∙10−3моль/л.
ƒл€ концентрированных растворов сильных электролитов, согласно первому приближению ƒеба€-’юккел€, при расчете водородного показател€ рЌ следует учитывать коэффициенты активности ионов g, которые завис€т от ионной силы раствора I:
I = 0,5 å(Ci ∙ zi 2) = 0,5 ∙ (2∙10−3 ∙ 12 + 6,67∙10−4 ∙ 22 + 3,33∙10−3 ∙ 12) = 7,99∙10−3,
−lgї 0,5 zi 2 ∙ 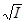 = 0,5∙ 12∙
= 0,5∙ 12∙  = 0,0446 или = 0,902
= 0,0446 или = 0,902
где Ci − мол€рна€ концентраци€ i -го иона, zi − зар€д i -го иона.
“огда величина рЌ равна рЌ = −lg = −lg  = −lg(2∙10−3∙ 0,902) = 2,74.
= −lg(2∙10−3∙ 0,902) = 2,74.
«адача 3. –асчетами доказать, будет ли образовыватьс€ осадок Ag2SO4 при смешивании 20 мл 5,0Ј10−4 ћ раствора нитрата серебра AgNO3 и 30 мл 1,0Ј10−7 ћ раствора сульфата натри€ Na2SO4. —тепень диссоциации веществ AgNO3 и Na2SO4 прин€ть равной 100%.
–ешение. ”словием выпадени€ осадка при проведении реакции в растворе €вл€етс€ превышение произведени€ концентрации ионов в конечном объеме смеси (ѕK) в соответствии со стехиометрическими коэффициентами над величиной произведени€ растворимости (ѕ–).
ѕри смешении растворов сильных электролитов (на что указывает значение степени диссоциации) AgNO3 и Na2SO4 может протекать реакци€ с образованием малорастворимого Ag2SO4
2AgNO3 + Na2SO4 → Ag2SO4 ↓+ 2NaNO3,
и при этом объем образовавшейс€ системы составл€ет
V смеси = V р-ра (AgNO3) + V р-ра (Na2SO4) = 20 + 30 = 50 мл
¬ соответствии с установившемс€ положением равновеси€ в насыщенном растворе труднорастворимого электролита Ag2SO4, выражение произведени€ растворимости имеет вид:
ѕ– (Ag2SO4) = [Ag+]2Ј[SO42−] = 1,2Ј10−5.
“огда выражение произведени€ концентрации ионов
ѕK =  .
.
“ак как соли AgNO3 и Na2SO4 €вл€ютс€ сильными электролитами со степенью диссоциации 100%, то мол€рна€ концентраци€ Ag+ в растворе AgNO3 составл€ет 5Ј10−4 моль/л
AgNO3 → Ag+ + NO3− ,
5Ј10−4 5Ј10−4 5Ј10−4
а концентраци€ SO42− в растворе Na2SO4 − 10−7 моль/л
Na2SO4 → 2Na+ + SO42−
10−7 2Ј10−7 10−7
–ассчитаем концентрации ионов Ag+ и SO42− в смеси двух растворов:
моль/л
моль/л
и подставим эти значени€ в выражение дл€ расчета ѕK = (2Ј10−4)2Ј6Ј10−6 = 2,4Ј10−13. ƒанное значение меньше ѕ–, следовательно, осадок Ag2SO4 не выпадет.
«адача 4. ¬ычислить значение рЌ раствора ацетата натри€ CH3COONa, полученного при растворении 4,1 г безводной соли в воде, если объем полученного раствора равен 100 см3. K дисс(—Ќ3—ќќЌ) = 1,75∙10Ц5,  = 10Ц14
= 10Ц14
–ешение. ¬ычислим мол€рную концентрации ацетата натри€ в полученном водном растворе:
|
|
|
= 0,5 моль/л
—оль CH3COONa образована сильным основанием NaOH и слабой кислотой CH3COOЌ (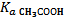 = 1,8∙10Ц5), поэтому гидролизуетс€ по аниону согласно сокращенному ионно-молекул€рному уравнению:
= 1,8∙10Ц5), поэтому гидролизуетс€ по аниону согласно сокращенному ионно-молекул€рному уравнению:
—Ќ3—ќќ− + Ќ2ќ ⇄ —Ќ3—ќќЌ + ќЌ−
Ќайдем значение константы гидролиза по формуле
K г = 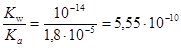 .
.
“ак как имеет место быть гидролиз по аниону, то
рЌ = 14 + 0,5lg(K г∙ C) = 14 + 0,5lg(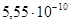 ∙ 0,5) = 9,22.
∙ 0,5) = 9,22.
«адачи дл€ самосто€тельного решени€
ѕримечание: все растворы, указанные в задачах, наход€тс€ при стандартной температуре; значени€ констант диссоциации и произведений растворимости электролитов приведены также при стандартной температуре.
«адачи 1−8. –ассчитать степень диссоциации слабого электролита в водном растворе и рЌ последнего, если известны мол€рна€ концентраци€ — раствора и константа диссоциации K д этого электролита.
| є | Ёлектролит | —, моль/л | K д | є | Ёлектролит | —, моль/л | K д |
| —Ќ3—ќќЌ | 0,010 | 1,8∙10−5 | HClO | 0,100 | 2,8∙10−8 | ||
| HCN | 0,001 | 4,9∙10−10 | C6H5COOH | 0,005 | 6,1Ј10 −5 | ||
| HNO2 | 0,500 | 5,1∙10−4 | HF | 0,200 | 6,6Ј10−4 | ||
| NH4OH | 0,005 | 1,7∙10−5 | NH4OH | 0,001 | 1,7∙10−5 |
«адачи 9−16. »спользу€ первое приближение ƒеба€-’юккел€, рассчитать рЌ водного раствора сильного электролита с мол€рной концентрацией —.
| є | Ёлектролит | —, моль/л | є | Ёлектролит | —, моль/л |
| HNO3 | 0,001 | NaOH | 0,100 | ||
| ClCH2COOH | 0,010 | KOH | 0,500 | ||
| HCl | 0,050 | Ba(OH)2 | 0,001 | ||
| HBr | 0,005 | CsOH | 0,010 |
«адачи 17−22. »спользу€ первое приближение ƒеба€-’юккел€, рассчитать активность ионов водорода и рЌ в водном растворе сильного электролита с мол€рной концентрацией —, содержащем также соль с одноименным ионом в концентрации 0,01 моль/л.
| є | Ёлектролит | —, моль/л | —оль | є | Ёлектролит | —, моль/л | —оль |
| HCl | 0,001 | NaCl | KOH | 0,100 | KCl | ||
| HNO3 | 0,015 | NaNO3 | Ba(OH)2 | 0,010 | BaCl2 | ||
| HBr | 0,005 | KBr | NaOH | 0,002 | NaNO3 |
«адачи 23−28. ќпределить, как и на сколько изменитс€ значение рЌ миллимол€рного раствора слабой кислоты с константой диссоциации K а, если к нему добавить такой же объем раствора соли с мол€рной концентрацией — (степень диссоциации соли прин€ть равной 100%).
| є | ислота | K а | —оль | —, моль/л |
| —Ќ3—ќќЌ | 1,8∙10−5 | —Ќ3—ќќK | 0,100 | |
| HNO2 | 5,1∙10−4 | NaNO2 | 0,002 | |
| Ќ—ќќЌ | 1,8∙10−4 | Ќ—ќќNa | 0,500 | |
| HClO | 2,8∙10−8 | KClO | 0,001 | |
| HCN | 4,9∙10−10 | LiCN | 0,005 | |
| HF | 6,6Ј10−4 | NaF | 0,010 |
«адачи 29−36. –ассчитать значение рЌ раствора, полученного смешением V 1 мл миллимол€рного раствора сильной кислоты и V 2 мл децимол€рного раствора сильного основани€. оэффициенты активности ионов прин€ть равными единице.
| є | ислота | V 1, мл | ќснование | V 2, мл |
| HNO3 | NaOH | |||
| HCl | Ba(OH)2 | |||
| HBr | KOH | |||
| ClCH2COOH | CsOH | |||
| HCl | LiOH | 0,5 | ||
| HNO3 | Ba(OH)2 | 1,5 | ||
| HBr | NaOH | |||
| ClCH2COOH | KOH |
«адачи 37−42. –ассчитайть мол€рную концентрацию слабого электролита с константой диссоциации K д в водном растворе, если известно значение рЌ последнего.
| є | Ёлектролит | K д | рЌ | є | Ёлектролит | K д | рЌ |
| —Ќ3—ќќЌ | 1,8∙10−5 | 5,5 | HClO | 2,8∙10−8 | 6,0 | ||
| NH4OH | 1,7∙10−5 | 9,5 | HCN | 4,9∙10−10 | 5,0 | ||
| HNO2 | 5,1∙10−4 | 3,5 | NH4OH | 1,7∙10−5 | 11,0 |
«адачи 43−50. –ассчитать количество сильного электролита со степенью диссоциации 90%, содержащегос€ в 500 мл водного раствора, если известно значение рЌ последнего. оэффициенты активности ионов прин€ть равными единице.
|
|
|
| є | Ёлектролит | рЌ | є | Ёлектролит | рЌ |
| HNO3 | 2,0 | NaOH | 11,5 | ||
| HCl | 4,5 | KOH | 10,0 | ||
| HBr | 3,0 | Ba(OH)2 | 13,0 | ||
| HNO3 | 4,2 | KOH | 12,5 |
«адачи 51−58. ћассова€ концентраци€ вещества в насыщенном водном растворе при 25 ∞C составл€ет — масс. ¬ычислить значение произведени€ растворимости ѕ– этого вещества при указанной температуре.
| є | ¬ещество | — масс, г/л | є | ¬ещество | — масс, г/л |
| Fe(OH)3 | 1,81∙10−9 | Mg(OH)2 | 6,44∙10−3 | ||
| Ag2CO3 | 3,20∙10−9 | Ag2SO4 | 8,36 | ||
| PbI2 | 6,22∙10−1 | Zn(OH)2 | 1,46∙10−4 | ||
| CaF2 | 1,68∙10−2 | CaCO3 | 6,93∙10−3 |
«адачи 59−66. –ассчитать значение рЌ насыщенного водного раствора малорастворимого гидроксида, если известна величина произведени€ растворимости ѕ– последнего.
| є | √идроксид | ѕ– | є | √идроксид | ѕ– |
| Mg(OH)2 | 5,5Ј10−12 | Cr(OH)3 | 6,7Ј10−31 | ||
| Fe(ќЌ)3 | 3,8Ј10−38 | Pb(OH)2 | 1,0Ј10−15 | ||
| Al(OH)3 | 5,1Ј10−33 | Zn(OH)2 | 1,3Ј10−17 | ||
| Cu(OH)2 | 5,0Ј10−19 | Bi(OH)3 | 3,0Ј10−32 |
«адачи 67−73. –асчетами доказать, будет ли образовыватьс€ осадок малорастворимой соли (известно еЄ значение произведени€ растворимости ѕ–), если к V 1 мл раствора вещества ј с мол€рной концентрацией — 1 добавить V 2 мл раствора вещества ¬ мол€рной концентрацией — 2? —тепень диссоциации веществ ј и ¬ прин€ть равной 100%.
| є | —оль | ѕ– | ¬ещество ј | V 1, мл | — 1, моль/л | ¬ещество ¬ | V 2, мл | — 2, моль/л |
| BaSO4 | 1,1Ј10−10 | BaCl2 | 0,020 | Na2SO4 | 0,100 | |||
| AgCl | 1,6Ј10−10 | AgNO3 | 0,001 | —а—l2 | 0,010 | |||
| SrSO4 | 3,2Ј10−7 | Sr(NO3)2 | 0,001 | Na2SO4 | 0,005 | |||
| PbI2 | 9,8 Ј10−9 | Pb(NO3)2 | 0,040 | KI | 0,001 | |||
| Ag2CO3 | 8,7Ј10−12 | AgNO3 | 0,002 | Na2CO3 | 0,010 | |||
| PbSO4 | 1,6Ј10−8 | Pb(NO3)2 | 0,010 | K2SO4 | 0,010 | |||
| ZnS | 7,4Ј10−27 | ZnCl2 | 0,005 | Na2S | 0,001 |
«адачи 74−79. –ассчитать растворимость соли (известно еЄ значение произведени€ растворимости ѕ–) в воде и в 0,005 ћ водном растворе вещества ј (степень диссоциации последнего и коэффициенты активности его ионов прин€ть равными 100% и 1 соответственно).
| є | —оль | ѕ– | ¬ещество ј |
| —а—ќ3 | 4,8Ј10−9 | —а—l2 | |
| PbSO4 | 1,6Ј10−8 | Na2SO4 | |
| AgBr | 6,3Ј10−13 | KBr | |
| CaC2O4 | 2,5Ј10−9 | Na2C2O4 | |
| ZnS | 7,4Ј10−27 | Na2S | |
| CaF2 | 4,0Ј10−11 | NaF |
«адачи 80−91. Ќаписать уравнение гидролиза по первой ступени соли в молекул€рной и ионно-молекул€рной формах. –ассчитать константу и степень гидролиза соли по этой ступени, если известны мол€рна€ концентраци€ соли — и значени€ констант кислотности K ai или констант основности K bi продукта полного гидролиза.
| є | —оль | —, моль/л | ѕродукт полного гидролиза | ||
| формула | значение K a(b)i | ||||
| K2SO3 | 0,005 | Ќ2SO3 | K аI = 1,7Ј10−2 K аII = 6,3Ј10−8 | ||
| NH4NO3 | 0,001 | NH4OH | K b= 1,7∙10−5 | ||
| Na2S | 0,004 | Ќ2S | K аI = 5,7Ј10−8 K аII =1,2Ј10 −15 | ||
| ZnCl2 | 0,002 | Zn(OH)2 | K bI = 4,4Ј10 −5 K bII = 1,5Ј10 −9 | ||
| —Ќ3—ќќK | 0,100 | —Ќ3—ќќH | K а= 1,8∙10−5 | ||
| Pb(NO3)2 | 0,060 | Pb(OH)2 | K bI = 9,6Ј10 −4 K bII = 3,0Ј10 −8 | ||
| K2SiO3 | 0,001 | H2SiO3 | K аI = 2,2Ј10 −10 K аII = 1,6Ј10 −12 | ||
| K2—ќ3 | 0,001 | Ќ2CO3 | K аI = 4,3Ј10 −7 K аII = 5,6Ј10 −11 | ||
| NaCN | 0,020 | HCN | K а= 4,9 Ј10 −10 | ||
| NH4—l | 0,010 | NH4OH | K b= 1,7∙10−5 | ||
| KNO2 | 0,001 | HNO2 | K а = 5,1∙10−4 | ||
| KЌ—ќ3 | 0,050 | Ќ2CO3 | K аI = 4,3Ј10 −7 K аII= 5,6Ј10 −11 | ||
|
|
|






