ѕримеры –ешени€ задач контрольной работы
ќсновы химической термодинамики
«адача 1
¬ычислить тепловой эффект химической реакции при 298 : 1) при – = const; 2) при V = const. “епловой эффект образовани€ веществ при стандартных услови€х найти по таблице.
4NO (г) + 6H2O (ж) = 4NH3 (г) + 5O2 (г)
ћетодика решени€
1. “ак как в условии задачи рекомендуетс€ применить теплоты образовани€ участников реакции, то дл€ решени€ задачи примен€ют первое следствие из закона √есса.
2. ‘ормулировка первого следстви€ из закона √есса: тепловой эффект химической реакции, протекающей при посто€нном давлении, равен сумме энтальпий образовани€ продуктов реакции за вычетом суммы энтальпий образовани€ исходных веществ с учетом стехиометрических коэффициентов.
3. ѕервое следствие из закона √есса в общем виде математически записываетс€ следующим образом
ΔH0 r,298 = ΣνiΔH0298, f (прод) Ц ΣνiΔH0298, f (исх)
где ΔH0 r,298 Ц тепловой эффект химической реакции;
ΣνiΔH0298, f (прод), ΣνiΔH0298, f (исх) алгебраическа€ сума теплот образовани€ продуктов и исходных веществ соответственно;
νi Ц число моль данного участника реакции.
4. ƒл€ рассматриваемой реакции
ΔH0 r,298 = (4ΔЌ0298, f (  ) + 5ΔЌ0298, f (
) + 5ΔЌ0298, f (  )) Ц (4ΔЌ0298, f (
)) Ц (4ΔЌ0298, f (  ) + 6ΔЌ0298, f (
) + 6ΔЌ0298, f ( 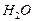 ))
))
5∞. »з таблицы выписываем термодинамические константы:
| вещество | ΔH0298, f, кƒж/моль |
| продукты NH3 (г) O2 (г) | Ц 46,19 |
| исходные вещества NO (г) H2O (ж) | 90,37 Ц285,84 |
ΔH0 r,298 = (4×(Ц 46,19) + 5×0) Ц (4×90,37 + 6× (Ц285,84)) =
= Ц 184,76 Ц (361,48 Ц 1715,04) = Ц 184,76 Ц (Ц1353,56) = 1168,8 кƒж.
ќбратите внимание на размерность величин
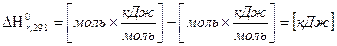
6. ѕо известному значению теплового эффекта реакции ΔH0 r,298 при посто€нном давлении можно рассчитать тепловой эффект реакции при посто€нном объеме QV = ΔU.
ѕри условии, что P = const и “ = const взаимосв€зь ΔH0 r,298 и ΔU определ€етс€ выражением
ΔH0 r,298 = Qp = ΔU +PΔV =ΔU + (PV2 Ц PV1) =ΔU + (n2RT Цn1RT) = ΔU + ΔnRT
ΔH0 r,298 = ΔU + ΔnRT (3.1.2),
где Δn Ц изменение числа молей газообразных продуктов реакции и исходных веществ
Δn = Σn (прод) Ц Σn (исх),
“аким образом тепловой эффект реакции при посто€нном объеме ΔU равен
ΔU = ΔH0 r,298 Ц ΔnRT
ƒл€ рассматриваемой реакции Δn = (4 + 5) Ц 4 = 5 моль.
7. ќбратите внимание на размерность энтальпии реакции и слагаемого ΔnRT:
 .
.
Ёнтальпи€ реакции 1168,8 кƒж должна быть переведена в ƒж.
ΔU=1168,8×103 Ц5×8,314×298 =1168800 Ц12387,86 = 1156412,14 = 1156,4 кƒж
ќтвет: ΔH0 r,298 = 1168,8 кƒж; ΔU = 1156,4 кƒж.
«адача 2
¬ычислите тепловой эффект реакции при температуре “:
|
|
|
4—ќ(г) + 2SO2(г) = S2(г) + 4CO2(г)
ћетодика решени€
1. “епловой эффект химической реакции, протекающей при р = const и температуре 500 , отличной от стандартной, рассчитывают по уравнению ирхгофа в интегральной форме:

2. ¬ыпишем термодинамические константы участников реакции из таблицы 1 ѕриложени€:
| вещество | ΔЌ0298, f, кƒж/моль | “еплоемкость, ƒж/моль× оэффициенты уравнени€ —0р=f(T) | “емпературный интервал | |||
| a | b×103 | c×10Ц5 | c×106 | |||
| S2(г) | 129,1 | 36,11 | 1,09 | Ц 3,52 | 273 Ц 2000 | |
| CO2(г) | Ц 393,51 | 44,14 | 9,04 | Ц 8,53 | 298 Ц 2500 | |
| —ќ(г) | Ц 110,5 | 28,41 | 4,10 | Ц 0,46 | 298 Ц 2500 | |
| SO2(г) | Ц 296,9 | 42,55 | 12,55 | Ц 5,65 | 298 Ц 1800 |
3. “епловой эффект реакции в стандартных услови€х рассчитываем по первому следствию из закона √есса:
ΔH0 r,298 = (1×ΔЌ0298, f (  ) + 4×ΔЌ0298, f (
) + 4×ΔЌ0298, f ( 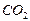 )) Ц (4×ΔЌ0298, f (
)) Ц (4×ΔЌ0298, f (  ) + 2×ΔЌ0298, f (
) + 2×ΔЌ0298, f (  ))
))
ΔH0 r,298 = (1 × 129,1 + 4 × (Ц 393,51)) Ц (4 × (Ц 110,5) + 2 × (Ц 296,9)) =
= (129,1 Ц 1574,04) Ц ((Ц 442) Ц 593,8) = Ц 1573,04 + 1035,8 =
= Ц 537,24 кƒж
4. –ассчитаем коэффициенты Δа, Δb, Δc, Δc', отражающие зависимость теплоемкости участников реакции от температуры, по уравнению:
Δа = Σni×ai(прод) Ц Σni×ai (исх) = (1 × 36,11 + 4 ×44,14) Ц (4 × 28,41 + 2 × 42,55) =
= (36,11 + 176,56) Ц (113,64 + 85,1) = 13,93 ƒж/
Δb = Σni×bi(прод) Ц Σni×bi (исх) = [(1 × 1,09 + 4 × 9,04) Ц (4 × 4,10 + 2 × 12,55)]×10Ц3 =
= [(1,09 + 36,16) Ц (16,4 + 25,1)] × 10Ц3 = Ц 4,25× 10Ц3 ƒж/
Δс = 0 (так как все вещества неорганические)
Δс' = Σni× с'i(прод) Ц Σni× с'i(исх) =
= [(1 × (Ц 3,52) + 4 ×(Ц 8,53)) Ц (4 × (Ц 0,56) + 2 ×(Ц 5,65))] × 105 = [(Ц3,52 Ц 34,12) Ц
Ц (Ц 2,24 Ц 11,3)]× 105 = [Ц37,65 + 13,54]× 105 = Ц24,11 × 105 ƒж/
5. ѕодставл€ем рассчитанные величины Δа, Δb, Δc, Δc' и определ€ем тепловой эффект химической реакции при 500 .
ќбратите внимание: размерность всех слагаемых должна быть одинаковой, дл€ этого ΔH0 r,298 умножаем на 103 и переводим таким образом из кƒж в ƒж.


ΔH0 r,500 меньше нул€, следовательно реакци€ экзотермическа€, идет с выделением тепла.
6. „тобы определить, насколько при данной температуре тепловой эффект при посто€нном давлении Qp отличаетс€ от теплового эффекта при посто€нном объеме Qv необходимо применить уравнение 1.2 (пункт 6∞ решени€ задачи 1).
Qp Ц Qv = ΔnRT
ΔH0 r,298 Ц ΔU = ΔnRT
где Δn Ц изменение числа молей газообразных продуктов реакции и исходных веществ Δn = Σn (прод) Ц Σn (исх).
ƒл€ данной реакции Δn = (1+4) Ц (4+2) = Ц1 моль
Qp Ц Qv = ΔH0 r,500 Ц ΔU = Ц1моль×8,314  ×500 = 4157 ƒж = 4,16 кƒж.
×500 = 4157 ƒж = 4,16 кƒж.
ќтвет: ΔH0 r,500 = Ц536,94 кƒж. Qp Ц Qv = 4,16 кƒж.
«адача 3
CO(г) + 3H2(г) = CH4(г) + H2O (г)
–ассчитать константу равновеси€ дл€ температур 298 и 1500 . —делать вывод о состо€нии равновеси€.
ћетодика решени€
1. ¬ыпишем табличные данные, необходимые дл€ решени€:
|
|
|
| ¬ещества | ΔH0298, f кƒж/моль | S0 298, f ƒж/мольЈ | —0– 298, ƒж/моль | Cp= f (T) | “емпературный интервал | |||
| а | bЈ10 3 | сЈ10 6 | с'Ј10 -5 | |||||
| —Ќ4(г) | Ц 74,85 | 186,19 | 35,79 | 17,45 | 60,46 | Ц 1,117 | - | 298-1500 |
| H2O (г) | Ц 241,84 | 188,74 | 33,56 | 30,00 | 10,71 | 0,33 | 298-2500 | |
| —ќ(г) | Ц110,5 | 197,4 | 29,15 | 28,41 | 4,10 | Ц0,46 | 298-2500 | |
| Ќ2(г) | 130,6 | 28,83 | 27,28 | 3,26 | - | 0,502 | 298-3000 |
2. ќпредел€ем изменение энтальпии при стандартных услови€х по первому следствию из закона √есса:
ΔH0 r,298.= ΣνiΔH0298, f(прод) Ц ΣνiΔH0298, f(исх)
ΔH0 r,298.= (1×(Ц74,85) +1×(Ц241,84)) Ц (1×(Ц110,5) + 3×0) = Ц206,19 кƒж =
= Ц206190 ƒж. ΔH0 r,298<0, следовательно реакци€ экзотермическа€.
3. Ёнтропи€ Ц это функци€, характеризующа€ меру беспор€дка. ¬ системе наход€тс€ только газообразные вещества, и в результате реакции количество молей убывает. —ледовательно, в результате реакции то энтропи€ снижаетс€.
оличественно изменение энтропии определим по формуле, аналогичной формуле первого следстви€ закона √есса:
ΔS0 r, 298.=ΣνiS0298, f (прод) Ц ΣνiS0298, f (исх)
ΔS0 r, 298.= (1×186,19 + 1×188,74) Ц (1×197,4 + 3×130,6) = 374,93 Ц 589,2
ΔS0 r, 298.= Ц214,27 ƒж/
4. ќпредел€ем изменение изобарно-изотермического потенциала (энергии √иббса) реакции по обобщенному уравнению первого и второго начал термодинамики:
ΔG0 r, 298= ΔH0 r,298 Ц T×ΔS0 r,298 = Ц206190 ƒж. Ц 298 ×(Ц214,27) ƒж/
ΔG0r, 298= Ц206190 + 63852,46 = Ц142337,5 ƒж = Ц142,34 кƒж
ΔG0r, 500= Ц206190 + 107135 = Ц99,055 кƒж
ѕротекание реакции в пр€мом направлении при данных услови€х термодинамически разрешено, поскольку ΔG0r, 298<0, ΔG0r, 500<0
4. ¬еличину р при “ = 298 и “ = 500 рассчитаем по уравнению изотермы реакции при стандартных услови€х.
ΔG0t= Ц RTln р
1n р= Ц(ΔG0t /RT);
1n р (298) = Ц (Ц214,39×103)/(8,31×298) = 86,5 р(298) = 3,8×1037
1n р (500) = Ц (Ц99,055×103)/(8,31×500) = 23,82 р(500) = 2,21×1010
ѕо величине р можно сделать вывод о состо€нии равновеси€ в системе. ¬ рассматриваемом случае и при “ = 298 и при “ = 500 константа равновеси€ р очень велика, следовательно, реакци€ протекает в пр€мом направлении и равновесие практически полностью смещено в сторону образовани€ продуктов. ”величение температуры вызывает незначительное смещение равновеси€ в сторону исходных веществ, что отвечает качественному правилу Ће Ўателье.
ќтвет: ΔH0 r,298 = Ц206,19 кƒж; ΔS0 r, 298.= Ц214,27 ƒж/ ; ΔG0r, 298 = Ц142,34 кƒж
ΔG0r, 500 = Ц99,055 кƒж; р(298) = 3,8×1037 ; р(500) = 2,21×1010
ћноговариантные задачи.
ќсновы химической термодинамики
«адача 1
¬ычислить тепловой эффект химической реакции при 298 : 1) при – = const; 2) при V = const. “епловой эффект образовани€ веществ при стандартных услови€х найти по таблице.
“аблица 1
| є варианта | –еакци€ | є ваиранта | –еакци€ |
| 2H2 + CO = CH3OH(∆) | SO2 + Cl2 = SOCl2 | ||
| 4HCl + O2 = 2H2O(∆) + 2Cl2 | CO + 3H2 = CH4 + H2O(∆) | ||
| NH4Cl(тв) = NH3 + HCl | 2CO + SO2 = Sромб + 2CO2 | ||
| 2N2 + 6H2O(∆)=4NH3 + 3O2 | CO + Cl2 = COCl2(√) | ||
| 4 NO +6H2O(∆)= 4NH3 + 5O2 | CO2 + H2 = CO + H2O(∆) | ||
| 2Nќ2 = 2NO+ O2 | CO2 + 4H2 = CH4 + 2H2O(∆) | ||
| N2ќ4 = 2Nќ2 | 2CO2 = 2CO + O2 | ||
| ћg(oH)2= ћgo+ H2O(√) | CH4 + CO2 = 2CO + 2H2 | ||
| —aCO3=CaO+CO2 | C2H6 = C2H4 + H2 | ||
| Ca(oH)2 = Cao+ H2O(√) | C2H5OH(∆) = C2H4 + H2O(∆) | ||
| Sромб+H2O(∆)=SO2+2H2 | CH3CHO(√) + H2 = C2H5OH(∆) | ||
| Sромб+2CO2 =SO2+2CO | C6H6(∆)+ 3H2 = C6H12 | ||
| 2SO2+O2 =2SO3 |
|
|
|
«адача 2
¬ычислите тепловой эффект реакции при температуре “:
“аблица 2
| ¬ариант | –еакци€ | “1, |
| 2SO2+O2 =2SO3(√) | ||
| 4CO + 2SO2 = S2 (√) + 4CO2 | ||
| CO + 3H2 = CH4 + H2O(√) | ||
| SO2 + Cl2 = SOCl2(√) | ||
| CO + Cl2 = COCl2(√) | ||
| CO2 + H2 = CO + H2O(√) | ||
| CO2 + 4H2 = CH4 + 2H2O(√) | ||
| 2CO2 = 2CO + O2 | ||
| CH4 + CO2 = 2CO + 2H2 | ||
| C2H6 = C2H4 + H2 | ||
| C2H5OH(∆) = C2H4 + H2O(∆) | ||
| CH3CHO(√) + H2 = C2H5OH(∆) | ||
| —ќ(г) + 2Ќ2(г) = —Ќ3ќЌ(г) | ||
| 4Ќ—1 + ќ2 = 2Ќ2ќ(г) + 2—l2 | ||
| NH4Cl(“¬) = NH3 + HCl | ||
| 2N2 + 6Ќ2ќ(г) = 4NЌ3 + 3O2 | ||
| 4NO + 6H2O(г) = 4NH3 + 5O2 | ||
| 2NO2 = 2NO + O2 | ||
| Mg(OH)2 = MgO + H2O | ||
| —aCO3=CaO+CO2 | ||
| N2ќ4 = 2Nќ2 | ||
| Ca(oH)2 = Cao+ H2O(√) | ||
 S2(г)+H2O(г)=SO2+2H2 S2(г)+H2O(г)=SO2+2H2
| ||
 S(г)+2CO2 =SO2+2CO S(г)+2CO2 =SO2+2CO
| ||
| NH4Cl(“¬) = NH3 + HCl |
«адача 3
–ассчитать константу равновеси€ дл€ температуры 1500 . —делать вывод о состо€нии равновеси€.
“аблица 3
| ¬ариант | –еакци€ |
| —ќ(г) + 2Ќ2(г) = —Ќ3ќЌ(г) | |
| CO + 3H2 = CH4 + H2O(√) | |
| 2N2 + 6Ќ2ќ(г) = 4NЌ3 + 3O2 | |
| NH4Cl(“¬) = NH3 + HCl | |
| —aCO3=CaO+CO2 | |
| N2ќ4 = 2Nќ2 | |
| Ca(oH)2 = Cao+ H2O(√) | |
| 4NO + 6H2O(г) = 4NH3 + 5O2 | |
| 2NO2 = 2NO + O2 | |
| Mg(OH)2 = MgO + H2O | |
 S2(г)+H2O(г)=SO2+2H2 S2(г)+H2O(г)=SO2+2H2
| |
 S(г)+2CO2 =SO2+2CO S(г)+2CO2 =SO2+2CO
| |
| 2SO2+O2 =2SO3(√) | |
| SO2 + Cl2 = SOCl2(√) | |
| 4Ќ—1 + ќ2 = 2Ќ2ќ(г) + 2—l2 | |
| 4CO + 2SO2 = S2 (√) + 4CO2 | |
| CO + Cl2 = COCl2(√) | |
| CO2 + H2 = CO + H2O(√) | |
| CO2 + 4H2 = CH4 + 2H2O(√) | |
| 2CO2 = 2CO + O2 | |
| CH4 + CO2 = 2CO + 2H2 | |
| C2H6 = C2H4 + H2 | |
| C2H5OH(∆) = C2H4 + H2O(∆) | |
| CH3CHO(√) + H2 = C2H5OH(∆) | |
| C6H6(∆)+ 3H2 = C6H12 |






