—амосто€тельна€ работа є 7
јЋ№ƒ≈√»ƒџ » ≈“ќЌџ
¬ариант 1
1. ƒайте названи€ следующим соединени€м:

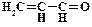
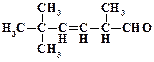
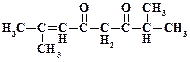
2. ѕриведите структурные формулы и дайте названи€ изомерных альдегидов состава C4H8O.
3. Ќапишите реакции пропионового альдегида с H2O [H+], NH2OH, NH2-NH-C6H5, CH3‑CH2-NO2 [OH-], PCl5, KMnO4 [H+ при нагревании].
4. Ќапишите уравнени€ реакций, назовите конечные (где необходимо - и промежуточные) продукты:
CH3-CHO + CH3OH [H+] → Е CH3OH [H+] → Е
2CH3CH2CH2CHO [NaOH изб.] → Е
(CH3)2C=C=O + CH3CH2OH → Е
CH3CH=CHCHO + HBr → Е
5. ќсуществите превращени€, назовите промежуточные и конечные продукты:
CH3CH2Br 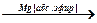 Е +
Е + 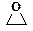 Ѓ Е
Ѓ Е  Е
Е 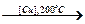 Е
Е
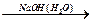 Е
Е  Е
Е  Е
Е
6. ”становите строение соединени€ C5H10O, которое дает положительную галоформную реакцию и конденсируетс€ с 2,4-динитрофенилгидразином, а при окислении в жестких услови€х преврашаетс€ в смесь кислот, одна из которых - пропионова€. ѕриведите уравнени€ упоминаемых реакций, назовите промежуточные продукты.
7. ѕредложите схему синтеза 2,3-диметил-2-бутанола из ацетона и неорганических реагентов.
—амосто€тельна€ работа є 7
јЋ№ƒ≈√»ƒџ » ≈“ќЌџ
¬ариант 2
1. ƒайте названи€ следующим соединени€м:
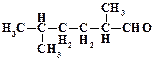
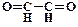

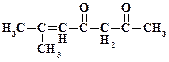
2. ѕриведите структурные формулы и дайте названи€ изомерных кетонов состава C4H8O.
3. Ќапишите реакции акролеина с H2O [H+], NH2OH, NH2-NH-C6H5, CH3-CH2-NO2 [OH-], PCl5, KMnO4 [при 0∞—].
4. Ќапишите уравнени€ реакций, назовите конечные (где необходимо - и промежуточные) продукты:
CH3-CH2-—O-CH3 + NaHSO3 → Е + HCl [H2O] → Е
CH3CHO + CH3NO2 [NaOH] → Е
(CH3)2C=CHCH3 + O3 → Е + H2O [Zn] → Е
CH3COCH2CH3 + 3Br2 [NaOH изб.] → Е
5. ќсуществите превращени€, назовите промежуточные и конечные продукты:
CH2=CHCHO 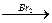 Е
Е 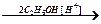 Е
Е 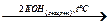 Е
Е  Е
Е 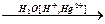 Е
Е
6. ”становите строение соединени€ C7H14O, способного к конденсации с 2,4-динитро≠фе≠нил≠гидразином, при гидрировании оно образует соединение C7H16O, которое после дегидратации и озонолиза дает смесь пропионового альдегида и метилэтилкетона. ѕриведите уравнени€ упоминаемых реакций, назовите промежуточные продукты.
7. ѕредложите схему синтеза транс -6-метил-3-циклогексен-карбальдегида из этанола и неорганических реагентов.
—амосто€тельна€ работа є 7
јЋ№ƒ≈√»ƒџ » ≈“ќЌџ
¬ариант 3
1. ƒайте названи€ следующим соединени€м:
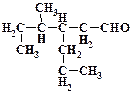
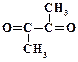
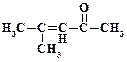

2. ѕриведите структурную формулу и дайте название альдегида состава C5H10O, не имеющего атомов водорода в α-положении к карбонильной группе.
3. Ќапишите реакции пропионового альдегида с 2CH3OH [H+], NH2-NH2, NaHSO3, CH3MgI/H2O[H+], CH3-CHO [OH-], циклопентаноном [OH-].
4. Ќапишите уравнени€ реакций, назовите конечные (где необходимо - и промежуточные) продукты:
(CH3)2CH-CHO + CH3MgI [эфир] → Е
|
|
|
CH3-CH2-CH2-—O-CH3 + PCl5 [t∞C] → Е
CH3CH2C≡CH + H2O [H+, Hg2+] → Е
(CH3CH2COO)2Ca [t∞C] → Е
5. ќсуществите превращени€, назовите промежуточные и конечные продукты:
CH3COOH 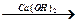 Е
Е  Е
Е  Е
Е 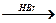 Е
Е 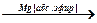 Е
Е 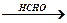 Е
Е  Е
Е 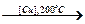 Е
Е 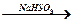 Е
Е
6. ”становите строение соединени€ C5H12O, реагирующего с металлическим натрием с выделением водорода, если известно также, что при нагревании с водным раствором перманганата кали€ оно образует соединение C5H10O, которое дает оксим и фенилгидразон. ѕосле дегидратации исходное соединение переходит в углеводород C5H10, одним из продуктов окислени€ которого €вл€етс€ ацетон. ѕриведите уравнени€ упоминаемых реакций, назовите промежуточные продукты.
7. ѕредложите схему синтеза трет-бутилглиоксал€ (CH3)3C-CO-CHOиз ацетона и неорганических реагентов.
—амосто€тельна€ работа є 7
јЋ№ƒ≈√»ƒџ » ≈“ќЌџ
¬ариант 4
1. ƒайте названи€ следующим соединени€м:
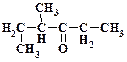

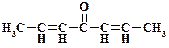

2. ѕриведите структурную формулу и дайте название метилкетона нормального строени€ состава C5H10O.
3. Ќапишите реакции акролеина с 2CH3OH [H+], NH2-NH2, NaHSO3, CH3MgI/H2O[H+], CH3-CHO [OH-], циклопентаноном [OH-].
4. Ќапишите уравнени€ реакций, назовите конечные (где необходимо - и промежуточные) продукты:
CH3CH2CHO + NH2OH → Е + P2O5 [t∞C] → Е
CH3-CH2-CH2-—O-CH2-CH3 + KMnO4 [H2SO4, t∞C] → Е
CH3CH2CH2OH [Cu, 200∞C] → Е
HC≡CH + C2H5OH [KOH] → Е + H2O [H+] → Е + C2H5OH
5. ќсуществите превращени€, назовите промежуточные и конечные продукты:
(CH3)2CHOH 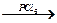 Е
Е 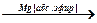 Е
Е  Е
Е  Е
Е
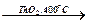 Е
Е 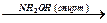 Е
Е
6. ”становите строение соединени€ C5H8O, дающего осадок с бисульфитом натри€ и реакцию серебр€ного зеркала, присоедин€ющего HBr. ≈сли ввести C5H8O в реакцию с гидразином, а полученный продукт прогреть с KOH, получитс€ углеводород C5H10, при озонолизе которого получаютс€ ацетон и уксусный альдегид. ѕриведите уравнени€ упоминаемых реакций, назовите промежуточные продукты.
7. ѕредложите схему синтеза 3-гексендиал€ из этанола и неорганических реагентов.






