ѕерва€ аналитическа€ группа ( +, Na+, NH  )
)
первой аналитической группе катионов относ€тс€ ионы кали€, натри€, а также ион аммони€ NH  . Ѕольшинство образуемых ими солей хорошо растворимо в воде. √руппового реагента, осаждающего все три катиона данной группы нет.
. Ѕольшинство образуемых ими солей хорошо растворимо в воде. √руппового реагента, осаждающего все три катиона данной группы нет.
–еакции катиона +
–еакции открыти€ катиона +, основанные на образование осадков, очень немногочисленные. ¬ажнейшие из них следующие.
1. ¬инна€ кислота Ќ2—4Ќ4ќ6 и ее кислота натриева€ соль Ц гидротартрат натри€ дают с растворами солей кали€ кристаллический осадок белого цвета

 KCl + NaHC4H4O6 =KHC4H4O6 + NaCl
KCl + NaHC4H4O6 =KHC4H4O6 + NaCl
K+ + HC4H4O  = KHC4H4O6 ↓
= KHC4H4O6 ↓
¬ыполнение опыта
¬ пробирку налейте 4-5 капель раствора соли кали€ и прибавьте столько же капель раствора гидротартрата натри€. ѕеремешайте содержимое пробирки стекл€нной палочкой Ц выпадает белый кристаллический осадок.
¬ывод:
2. атион + плам€ горелки окрашивает в бледно-фиолетовый цвет.
–еакции катиона Na+
Ѕольшинство солей натри€ хорошо растворимо в воде. ѕоэтому реакции, основанные на образовании труднорастворимых осадков, дл€ иона Na+ весьма немногочисленны.
√ексагидроксоантимонат кали€, дает с растворами солей натри€ белый кристаллический осадок гексагидроксоантимонат натри€.

 NaCl + K[Sb(OH)6] = Na[Sb(OH)6] + KCl
NaCl + K[Sb(OH)6] = Na[Sb(OH)6] + KCl
Na+ + [Sb(OH)6]- = Na[Sb(OH)6]

 ¬ыполнение опыта
¬ыполнение опыта
¬ пробирку налейте 5-6 капель соли натри€ и прибавьте такой же объем раствора гексагидроксоантимоната кали€ и потрите стенки пробирки стекл€нной палочкой. ¬ыпадает белый кристаллический осадок.
–еакции катиона NH 
1. ≈дкие щелочи разлагают соли аммони€, с выделением газообразного аммиака, который можно обнаружить по запаху.
NH4Cl + NaOH  NaCl + NH3↑ + H2O
NaCl + NH3↑ + H2O
NH  + OH-
+ OH-  NH3↑ + H2O
NH3↑ + H2O
¬ыполнение опыта
ѕоместите 1-2 мм раствора соли аммони€ в пробирку, добавьте 2-3 мл растра щелочи и нагрейте до кипени€, при этом выдел€етс€ газ с резким запахом. јммиак можно обнаружить также и по посинению влажной красной лакмусовой бумажки, поднесенной к отверстию пробирки. Ќи один из других катионов не дает аналогичной реакции.
¬ывод:
¬тора€ аналитическа€ группа (Ag+, Pb2+, Hg  )
)
–еакции катиона Ag+
’ромат кали€ дает с катионом Ag+ осадок хромата серебра кирпично-красного цвета.
2AgNO3 + K2CrO4 = Ag2CrO4↓ + 2KNO3
2Ag+ +.... →
¬ыполнение опыта
¬ пробирку возьмите 2-3 капли раствора нитрата серебра, добавьте 3-4 капли дистиллированной воды и 1-2 капли хромата кали€. ќбратите внимание на цвет
¬ывод:
–еакции катиона Pb2+
1. ’ромат кали€ образует малорастворимый хромат свинца желтого цвета.
Pb(NO3)2 + K2CrO4 = PbCrO4↓ + 2KNO3
Pb2+ +..... →
¬ыполнение опыта
¬ пробирку налейте 3-4 капли раствора нитрата синца и 2-3 капли раствора хромата кали€.
2. »одид кали€ образует и ионами свинца осадок желтого цвета.
Pb(NO3)2 + 2KI = PbI2 + 2KNO3

 Pb2+ +..... →
Pb2+ +..... →
¬ыполнение опыта
2-3 капл€м раствора нитрата свинца, помещенным в пробирку, прилейте 3-5 капель раствора иодида кали€.
¬ывод:
“реть€ аналитическа€ группа катионов (Ba2+, Sr2+, Ca2+)
|
|
|
–еакции катиона ¬а2+
ќсобо характерных реакций на катион ¬а2+ указать трудно, но наиболее широко используют следующие.
1. ’ромат кали€ и дихромат кали€ дают с катионам ¬а2+ осадок хромата бари€ желтого цвета.

 BaCl2 + K2CrO4 = BaCrO4 + 2KCl
BaCl2 + K2CrO4 = BaCrO4 + 2KCl
Ba2+ +... →
. ¬ыполнение опыта
¬ пробирку поместите 3 капли раствора хлорида (или нитрата) бари€, добавьте каплю раствора хромата кали€, перемешайте стекл€нной палочкой. ѕолученный осадок испытайте на растворимость в сол€ной и уксусной кислотах.
¬ывод:
2. ќксалат аммони€ (и другие растворимые соли щавелевой кислоты) образует с катионом ¬а2+ белый кристаллический осадок

 BaCl2 + (NH4)2C2O4 = BaC2O4 + 2NH4Cl
BaCl2 + (NH4)2C2O4 = BaC2O4 + 2NH4Cl
Ba2+ +.... →...
¬ыполнение опыта
¬ пробирку налейте 3 капли раствора соли бари€ (хлорида или нитрата) и прилейте 3-4 капли раствора оксалата аммони€. ѕолученный осадок разделите на 2 части и прилейте к одной части сол€ную кислоту, а к другой Ц уксусную. „то наблюдаетс€?
¬ывод:
3. —оли бари€ окрашивают бесцветное плам€ в желто-зеленый цвет.
–еакции катиона —а2+
ƒл€ открыти€ катиона —а2+ используют общеаналитические реакции, из которых следует отметить реакцию с оксалатом аммони€.

 CaCl2 + (NH4)2C2O4 = CaC2O4 + 2NH4Cl
CaCl2 + (NH4)2C2O4 = CaC2O4 + 2NH4Cl
—а2+ +... →
¬ыполнение опыта
¬ пробирку налейте 3 капли раствора хлорида кальци€ (или нитрата кальци€), прибавьте 3-4 капли оксалата аммони€. ѕолученный осадок испытайте на растворимость в сол€ной и уксусной кислотах.
¬ывод:
„етверта€ аналитическа€ группа катионов (Al3+, Zn2+, Cr3+, Sn2+)
–еакции катиона Cr3+
’ром образует два р€да устойчивых солей: соли хрома (III) и соли хромовой и двухромовой кислот. –астворы солей, содержащие катион Cr3+, имеют зеленую или фиолетовую окраску, растворы хроматов (CrO  ) Ц желтую, растворы дихроматов Ц (Cr2O
) Ц желтую, растворы дихроматов Ц (Cr2O 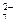 ) Ц оранжевую окраску.
) Ц оранжевую окраску.
ƒл€ ионов хрома характерны реакции окислени€ Ц восстановлени€ Cr(III) 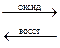 Cr(VI)
Cr(VI)
–еакции окислени€ Cr(III) в Cr(VI) можно проводить в щелочной и кислой средах
1. ќкисление в щелочной среде чаще всего провод€т пероксидом водорода (пероксидом натри€) или бромной (хлорной) водой.
Cr2(SO4)3 + 3H2O2 + 10NaOH = 2Na2CrO4 + 3Na2SO4 + 8H2O
 2 Cr3+ + 8OH- - 3ē → CrO
2 Cr3+ + 8OH- - 3ē → CrO  + 4H2O
+ 4H2O
3 H2O2 + 2ē → 2OH-
 2 Cr3+ + 16OH- + 3H2O2 = 2CrO
2 Cr3+ + 16OH- + 3H2O2 = 2CrO  + 8H2O + 6OH-
+ 8H2O + 6OH-
2Cr3+ + 10OH- + 3H2O2 = 2CrO  +8H2O
+8H2O
¬ыполнение опыта
2-3 капл€м раствора соли хрома (III), помещенным в пробирку, приливайте по капл€м раствор NаOH до растворени€ образовавшегос€ осадка, затем добавьте 2-3 капли Ќ2ќ2 и нагрейте. ќбратите внимание на измене цвета растворов.
¬ывод:






