«аселение атомных орбиталей электронами. ѕравило минимума энергии, принцип ѕаули и правило ’унда
«аселение атомных орбиталей электронами определ€етс€ правилом минимума энергии, принципом ѕаули и правилом ’унда.
Ёлектроны засел€ют атомные орбитали, начина€ с подуровн€ с меньшей энергией. ¬ этом состоит правило минимума энергии. ѕоследовательность в нарастании энергии подуровней акова: 1 s < 2 s < 2 p < 3 s < 3 p < 4 s ≤ 3 d < 4 p < 5 s и так далее Е
—огласно расчетам, электрон движетс€ не по какой-то определенной траектории, а может находитьс€ в любой части около€дерного пространства - т.е. можно говорить лишь о веро€тности (возможности) его нахождени€ на определенном рассто€нии от €дра.
Ёлектроны в атоме занимают самые энергетически выгодные атомные орбитали (орбитали с минимальной энергией), образу€ электронные облака определенной формы.
¬ случае s -орбитали электронное облако сферическое:
¬ случае p -орбиталей форма электронного облака гантелеобразна€:  ...
...
¬нутри атомных орбиталей веро€тность нахождени€ электронов велика; иными словами, имеетс€ высока€ электронна€ плотность. ѕространство вне объема орбиталей соответствует малой электронной плотности.
¬ каждой атомной орбитали может размещатьс€ максимально два электрона (принцип ѕаули).
ѕри наличии орбиталей с одинаковой энергией (например, трех р -орбиталей одного подуровн€) кажда€ орбиталь заполн€етс€ вначале наполовину (и поэтому на р -подуровне не может быть более трех неспаренных электронов), а затем уже полностью, с образованием электронных пар (правило ’унда).
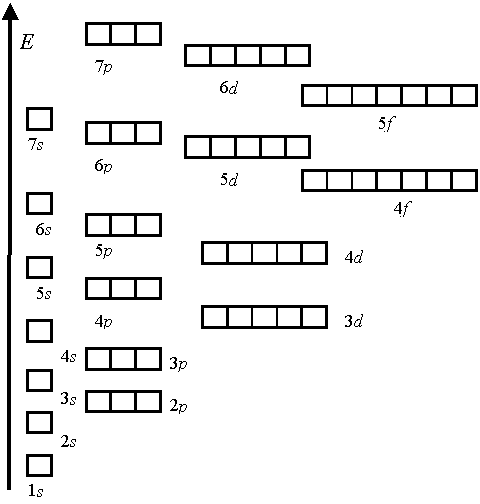
ƒл€ изображени€ электронной конфигурации атома нужно распределить его электроны по подуровн€м так, чтобы каждой атомной орбитали соответствовала одна квантова€ €чейка, и в соответствии с трем€ указанными правилами заселени€.
Ёлектронные конфигурации атомов
Ёлектронные конфигурации атомов записываютс€ в виде полных и сокращенных электронных формул:
1H 1 s 1
2He 1 s 2
3Li 1 s 22 s 1 = [2He] 2 s 1
4Be 1 s 22 s 2 = [2He] 2 s 2
5B 1 s 2 2 s 2 2 p 1 = [2He] 2 s 2 2 p 1
6C 1 s 2 2 s 2 2 p 2 = [2He] 2 s 2 2 p 2
7N 1 s 2 2 s 2 2 p 3 = [2He] 2 s 2 2 p 3
8O 1 s 2 2 s 2 2 p 4 = [2He] 2 s 2 2 p 4
9F 1 s 2 2 s 2 2 p 5 = [2He] 2 s 2 2 p 5
10Ne 1 s 2 2 s 2 2 p 6 = [2He] 2 s 2 2 p 6
11Na 1 s 2 2 s 2 2 p 6 3 s 110Ne] 3 s 1
12Mg 1 s 2 2 s 2 2 p 6 3 s 2 = [10Ne] 3 s 2
13Al 1 s 2 2 s 2 2 p 6 3 s 2 3 p 1 = [10Ne] 3 s 2 3 p 1
14Si 1 s 2 2 s 2 2 p 6 3 s 2 3 p 2 = [10Ne] 3 s 2 3 p 2
15P 1 s 2 2 s 2 2 p 6 3 s 2 3 p 3 = [10Ne] 3 s 2 3 p 3
16S 1 s 2 2 s 2 2 p 6 3 s 2 3 p 4 = [10Ne] 3 s 2 3 p 4
17Cl 1 s 2 2 s 2 2 p 6 3 s 2 3 p 5 = [10Ne] 3 s 2 3 p 5
18Ar 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 = [10Ne] 3 s 2 3 p 6
19K 1 s 2 2 s 2 2 p 6 3 s 2 3 p 64 s 1 = [18Ar] 4 s 1
20Ca 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 = [18Ar] 4 s 2
21Sc 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 1 4 s 2 = [18Ar] 3 d 1 4 s 2
22Ti 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 2 4 s 2 = [18Ar] 3 d 2 4 s 2
23V 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 3 4 s 2 = [18Ar] 3 d 3 4 s 2
24Cr 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 5 4 s 1 = [18Ar] 3 d 5 4 s 1
25Mn 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 5 4 s 2 = [18Ar] 3 d 5 4 s 2
26Fe 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 6 4 s 2 = [18Ar] 3 d 6 4 s 2
27Co 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 7 4 s 2 = [18Ar] 3 d 7 4 s 2
28Ni 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 8 4 s 2 = [18Ar] 3 d 8 4 s 2
29Cu 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 1 = [18Ar] 3 d 10 4 s 1
30Zn 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 = [18Ar,3 d 10] 4 s 2
31Ga 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 1 = [18Ar, 3 d 10] 4 s 2 4 p 1
32Ge 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 2 = [18Ar,3 d 10] 4 s 2 4 p 2
33As 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 3 = [18Ar,3 d 10] 4 s 2 4 p 3
34Se 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 4 = [18Ar,3 d 10] 4 s 2 4 p 4
35Br 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 5 = [18Ar,3 d 10] 4 s 2 4 p 5
36Kr 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 6 = [18Ar,3 d 10] 4 s 2 4 p 6
|
|
|
»з рассмотрени€ электронных конфигураций атомов видно, что элементы VIIIј-группы (He, Ne, Ar и другие) имеют завершенные s - и p - подуровни (s 2 p 6). “акие конфигурации обладают повышенной устойчивостью и обеспечивают химическую пассивность благородных газов.
¬ атомах остальных элементов внешние s - и p - подуровни - незавершенные, например у хлора: 17Cl = [10Ne] 3 s 2 3 p 5. Ќезавершенные подуровни и электроны на них называютс€ также валентными, поскольку именно они могут участвовать в образовании химических св€зей между атомами.
ѕравило лечковского (также ѕравило n+l; также используетс€ название правило ћаделунга) Ч эмпирическое правило, описывающее энергетическое распределение орбиталей в многоэлектронных атомах.
«аполнение электронами орбиталей в атоме происходит в пор€дке возрастани€ суммы главного и орбитального квантовых чисел 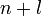 . ѕри одинаковой сумме раньше заполн€етс€ орбиталь с меньшим значением . ѕри одинаковой сумме раньше заполн€етс€ орбиталь с меньшим значением 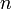 . .
|
ѕравило n+l предложено в 1936 г. немецким физиком Ё. ћаделунгом; в 1951 г. было вновь сформулировано ¬. ћ. лечковским.






