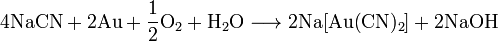—инильна€ кислота. Ѕесцветна€ легка€ низкокип€ща€ жидкость; ассоцииро-
вана за счет водородных св€зей (при комнатной температуре степень ассо-
циации равна 2). —уществует в двух таутомерных формах: нормальной (HЧ
C ≡ N:) и изо-форме (HЧN ≡ C:); при 25◦ C в равновесной смеси 0,5%
изо-формы, при охлаждении количесиво изо-формы уменьшаетс€. –азлагает-
с€ при сильном нагревании и на свету (образуютс€ формиат аммони€, ща-
велева€ кислота и бурый взрывоопасный осадок неустановленного состава).
Ќеограниченно смешиваетс€ с водой, про€вл€ет слабые кислотные свойства,
раствор называетс€ циановодородной кислотой. ¬ концентрированном раство-
ре неустойчив и постепенно разлагаетс€ с образованием формиата аммони€
(ингибитор Ч следы серной кислоты). Ќейтрализуетс€ щелочами. ѕро€вл€-
ет восстановительные свойства; сгорает на воздухе, реагирует с галогена-
ми, концентрированной серной кислотой, диоксидом азота. ∆идкий HCN Ч
пол€рный протонный растворитель с высокой диэлектрической проницаемо-
стью. ѕолучение см. 2027, 2037, 2123−5, 8396.
¬ насто€щий момент есть три наиболее распространенных метода получени€ синильной кислоты в промышленных масштабах:
ћетод јндрусова: пр€мой синтез из аммиака и метана в присутствии воздуха и платинового катализатора при высокой температуре:
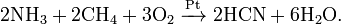
ћетод BMA: пр€мой синтез из аммиака и метана в присутствии платинового катализатора при высокой температуре:
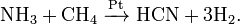
2C + H2 +N2 = 2HCN (выше 1800◦ C).
CO+NH3 = HCN+H2O
Mr = 27, 03; d = 0, 699(22); tпл = −13, 3◦ C; tкип = +25, 65◦ C.
1. HCN(разб.)+H2O(хол.) H3O+ +CN−; pKк = 9, 31,
HCN(конц.)+2H2O NH+4 +HCOO−.
2. HCN + NaOH(конц.)= NaCN+H2O.
3. HCN + NH3 Ј H2O(конц.) NH4CN + H2O (комн.).
4. HCN(г) +H2O+H2SO4(конц.)= CO+NH4HSO4.
5. HCN + 4H0(Zn, разб. HCl) = CH3NH2.
6. 4HCN + 5O2 = 4CO2 + 2N2 + 2H2O (сгорание на воздухе),
4HCN + O2 = 2C2N2 + 2H2O (150◦ C, кат. Ag).
7. HCN(р) +Cl2 (CN)Cl +HCl,
2HCN(г) +Cl2 = C2N2 + 2HCl (кат. активный уголь),
HCN + H2O+Cl2 = HOCN+2HCl (кат. Al2O3).
8. 2HCN + 5HClO = 2CO2↑ +H2O+N2↑ +5HCl.
9. 2HCN + NO2 = C2N2 +NO+H2O (комн.).
—оли синильной кислоты называютс€ цианидами. ÷ианиды подвержены сильному гидролизу. ѕри хранении водных растворов цианидов при доступе диоксида углерода они разлагаютс€:
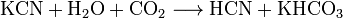
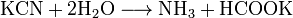
»он CN− (изоэлектронный молекуле —ќ) входит как лиганд в большое число комплексных соединений d-элементов. омплексные цианиды в растворах очень стабильны.
÷ианиды т€жЄлых металлов термически неустойчивы; в воде, кроме цианида ртути (Hg(CN)2), нерастворимы. ѕри окислении цианиды образуют соли Ч цианаты:
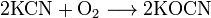
ћногие металлы при действии избытка цианида кали€ или цианида натри€ дают комплексные соединени€, что используетс€, например, дл€ извлечени€ золота и серебра из руд: