ѕреобладание того или иного процесса определ€етс€ общей константой совмещенного равновеси€, котора€ равна отношению произведени€ констант диссоциативного типа (т. е. ѕ–, ионизации, нестойкости комплекса) веществ, сто€щих в левой части уравнени€, к произведению констант диссоциативного типа веществ, сто€щих в правой части уравнени€. ѕри наличии стехиометрических коэффициентов, константы возвод€тс€ в степени, равные этим коэффициентам. онстанта равновеси€ (см.раздел 3.2.) показывает степень превращени€ исходных веществ в продукты. ≈сли в состо€нии равновеси€ преобладают продукты, константа равновеси€ имеет значение больше 1 равн> 1, равновесие смещено вправо, в сторону пр€мой реакции.» наоборот, если в состо€нии равновеси€ преобладают исходные вещества, константа равновеси€ имеет значение меньше 1 Kравн < 1, равновесие смещено влево, в сторону обратной реакции. †— точки зрени€ термодинамики при значени€х ΔG0, наход€щихс€ в диапазоне от -30кƒж/моль до 30кƒж/моль при изменении концентрации возможно изменение знака ΔG0 с минуса на плюс и наоборот. “акие реакции €вл€ютс€ обратимыми. »м соответствуют значени€ равн, наход€щиес€ в диапазоне от 10Ц5 до 105. «начени€ констант, выход€щие за пределы этого диапазона, соответствуют практически необратимым реакци€м. –еакции с равн> 1Ј105, протекают только в пр€мом направлении, а реакции с Kравн < 1Ј10Ц5 идут только в обратном направлении.
ѕримеры однотипных конкурирующих равновесий: –астворение осадка с образованием другого осадка
«апишем молекул€рное уравнение реакции
Pb—l2(к) + 2KI ⇄PbI2(к) + 2 —l
«апишем полное ионное уравнение
PbCI2(к) + 2 ++2I-⇄PbI2(к) + 2 ++ 2Cl-
«апишем сокращенное ионное уравнение
PbCl2(к) + 2I-⇄PbI2(к) + 2Cl- (1)
»сход€ из определени€ константы совмещенного равновеси€, можно сразу записать, чему будет она будет равна. Ёто будет отношение ѕ– осадка в левой части уравнени€ к ѕ– осадка в правой части уравнени€.
равн = ѕ–(PbCl2)/ѕ–(PbI2)
ƒл€ того, чтобы вывести константу совмещенного равновеси€ запишем константу равновеси€ данной реакции по закону действующих масс (в числителе сто€т равновесные концентрации продуктов, в знаменателе равновесные концентрации исходных веществ, возведенные в степень стехиометрических коэффициентов, см. раздел 3.2.). онцентраци€ твердых веществ считаетс€ величиной посто€нной и в константу равновеси€ не пишетс€. ѕишем только концентрации ионов.
равн =[Cl-]2/[I-]2. ƒалее домножим и поделим на концентрацию конкурирующего иона [Pb2+]
равн= [Pb2+][Cl-]2/[Pb2+][I-]2 . ¬ числителе и знаменателе получились ѕ– дл€ солей соответственно из левой и правой частей уравнени€ (1)
равн= ѕ–(PbCl2)/ѕ–(PbI2)==2,4·10-4/8,7·10-9 =2,7·104
равн> 1, близкак 1Ј105, осадок PbCI2растворитьс€ при добавлении избытка раствора KI.ѕроисходит конкуренци€ за общий ион Pb2+. Ђ¬ыигрываетї тот малорастворимый электролит, который имеет меньшее значение ѕ–.
ѕрисутствие в биологических жидкост€х большого числа ионов приводит к тому, что одновременно могут образовыватьс€ несколько малорастворимых электролитов. ¬ общем случае катион ћ+ может образовывать два малорастворимых электролита с анионами јЦ и ¬Ц: ћј и ћ¬. ѕри ѕ–(MA) = ѕ–(MB) и равных исходных концентраци€х јЦ и ¬Ц будет происходить одновременное образование ћј и ћ¬ в равных количествах. ѕри ѕ– (MA) > ѕ–(MB) и сопоставимых концентраци€х анионов происходит преимущественное образование ћ¬. ќтсюда следует, что чем меньше ѕ–, тем раньше (т.е. при меньшей концентрации) начнет выпадать осадок.
|
|
|
—равнение значений ѕ– возможно, если электролиты дают при диссоциации одинаковое число ионов. Ќапример:
а) AgI, AgCl; б) CaSO4, BaSO4; в) Ag2CrO4, Ag2CO3; г) PbCl2, PbI2, д) Ca3(PO4)2, Ba3(PO4)2,Mg3(PO4)2.
онкурирующие равновеси€ разных типов:
ј) равновесие с образованием комплексных соединений;
Ѕ) кислотно-основное равновесие;
ј) ¬ли€ние равновеси€ с образованием комплексного соединени€ на гетерогенное равновесие. „ем прочнее образуетс€ комплексное соединение, т.е.чем меньше нест. (см. раздел 10.3.), тем больше будет сдвинуто равновесие в сторону образовани€ комплекса, т.е. растворени€ осадка.
AgCl(к) ⇄ Ag+ + Cl Ц (насыщ р-р) ѕ–(AgCl) = 1,6 . 10Ц10
[Ag(NH3)2] + ⇄ Ag+ + 2NH3
нест.[Ag(NH3)2]+ = [Ag+]. [NH3]2 /[[Ag(NH3)2]+] =5,89.10Ц8
«апишем молекул€рное уравнение реакции
AgClкр.↓ + 2NH3 ↔ [Ag(NH3)2] Cl
«апишем сокращенное ионное уравнение
AgClкр.↓ + 2NH3 ↔ [Ag(NH3)2]+p-p + ClЦ (2)
»сход€ из определени€ константы совмещенного равновеси€, можно сразу записать, чему будет она будет равна. Ёто будет отношение ѕ– осадка левой части уравнени€ к нест комплексного иона в правой части уравнени€.
равн= ѕ–(AgCl)/ нест.[Ag(NH3)2]+
ƒл€ того, чтобы вывести константу совмещенного равновеси€ запишем константу равновеси€ данной реакции по закону действующих масс (в числителе сто€т равновесные концентрации продуктов, в знаменателе равновесные концентрации исходных веществ, возведенные в степень стехиометрических коэффициентов). онцентраци€ твердых веществ считаетс€ величиной посто€нной и в константу равновеси€ не пишетс€. ѕишем только концентрации ионов и молекул аммиака.
равн = [[Ag(NH3)2]+] .[ClЦ ] / [NH3]2. ƒалее домножим и поделим на концентрацию конкурирующего иона [Ag+]
равн = [[Ag(NH3)2]+] .[ClЦ ] †. [Ag+] /[NH3]2 [Ag+]
¬ числителе
ѕ–(AgCl) = [Ag+].[ClЦ ] †
¬ знаменателе
нест.[Ag(NH3)2]+ = [Ag+]. [NH3]2 / [[Ag(NH3)2]+]
равн = ѕ–(AgCl)/ нест.[Ag(NH3)2]+ = 1,6 . 10Ц10 /5,89.10Ц8 = 2,6.10Ц3
Kравн < 1, но при этом Kравн > 1Ј10Ц5, если через насыщенный раствор AgCl пропустить избыток аммиака, то это вызовет образование комплексного иона [Ag(NH3)2]+ и осадок AgCl растворитьс€. онкуренци€ за ион Ag +.
Ѕ) ¬ли€ние кислотно-основного равновеси€ на гетерогенное равновесие. –астворимость труднорастворимых солей, образованных анионами слабых кислот, значительно зависит от рЌ раствора. Ёто обусловлено возникающими конкурирующими процессами за анион слабой кислоты между ионом металла и Ќ+. ѕри определенном значении рЌ может произойти полное растворение осадка.
ѕример1. ќксалат кальци€ раствор€етс€ в сол€ной кислоте.
1. ќхарактеризуем константами диссоциативного типа все вещества, участвующие в равновесии оксалата кальци€ и щавелевую кислоту:
CaC2O4(к) ↔ Ca2+ + C2O42Ц(насыщ р-р);
|
|
|
ѕ–(CaC2O4) = 2,3Ј10Ц9
ўавелева€ кислота €вл€етс€ двухосновной кислотой, диссоциирует в две ступени и кажда€ ступень характеризуетс€ своей константой ионизации:
I.H2C2O4 ↔ H+ + ЌC2O4Ц;
K1(H2C2O4) =[ H+ ] ∙[ ЌC2O4Ц]/[ H2C2O4]=5,4 ∙10-2
II. ЌC2O4Ц ↔ H+ + C2O42Ц;
K2(H2C2O4) = [ H+ ] ∙[ C2O42-]/[ ЌC2O4Ц]=5,4 ∙10-5
—уммарное уравнение ионизации:
H2C2O4 ↔ 2H+ + C2O42-;
’арактеризуетс€ произведением двух констант ионизации:
K1(H2C2O4) ∙ K2(H2C2O4) =[ H+]2 ∙[ C2O42-]/[ H2C2O4]
2. «апишем уравнени€ реакций взаимодействи€ оксалата кальци€ с сол€ной кислотой в молекул€рном виде
CaC2O4(к) + 2HCl ⇄ CaCl2 + H2C2O4
«апишем полное ионное уравнение
CaC2O4(к) + 2H++ 2Cl- ⇄ Ca2+ + 2Cl-+ H2C2O4
«апишем сокращенное ионное уравнение
CaC2O4(к) + 2H+ ⇄ Ca2+ + H2C2O4 (3)
«апишем константу совмещенного равновеси€ исход€ из ее определени€
равн= ѕ–(CaC2O4) / K1(H2C2O4) ∙ K2(H2C2O4)
3. ¬ыведем и рассчитаем общую константу совмещенного равновеси€ дл€ реакции(3)
равн= [ ЌC2O4Ц] ∙[ Ca2+ ] /[ H+]2
ƒалее домножим и поделим на концентрацию конкурирующего иона [ C2O42-]
равн= [ ЌC2O4Ц] ∙[ Ca2+ ] ∙[ C2O42-] /[ H+]2∙[ C2O42] = ѕ–(CaC2O4) / K1(H2C2O4) ∙ K2(H2C2O4) = 2,3Ј10Ц9/ 5,4 ∙10-2Ј5,4 ∙10-5 =7,7Ј 10-4
Kравн< 1 близка к 1Ј10Ц5 такие реакции в стандартных услови€х не идут, поэтому в физиологических услови€х почка образует нерастворимый оксалат кальци€. Ќо оксалат кальци€ можно растворить in vitro в растворе сол€ной кислоты большой концентрации.
ѕример 2. ќксалат кальци€ не раствор€етс€ в уксусной кислоте
1. ќхарактеризуем константами диссоциативного типа все вещества, участвующие в равновесии оксалат кальци€, щавелевую кислоту и уксусную кислоту:
CaC2O4(к) ↔ Ca2+ + C2O42Ц (насыщ р-р);
ѕ–(CaC2O4) = 2,3Ј10Ц9
I.H2C2O4 ↔ H+ + ЌC2O4Ц; K1(H2C2O4) = 5,4 ∙10-2
II. ЌC2O4Ц ↔ H+ + C2O42Ц; K2(H2C2O4) = 5,4 ∙10-5
—уммарное уравнение ионизации:
H2C2O4 ↔ 2H+ + C2O42-;†
’арактеризуетс€ произведением двух констант ионизации:
K1(H2C2O4) ∙ K2(H2C2O4) =[ H+]2 ∙[ C2O42-]/[ H2C2O4]
CH3COOH ↔ H+ + CH3COOЦ;
K(CH3COOH) = [H+] ∙[CH3COO-]/[ CH3COOH]=1,74Ј10Ц5
2. «апишем уравнени€ реакций взаимодействи€ оксалата кальци€ с уксусной кислотой.
CaC2O4(к) + 2CH3COOH ⇄ Ca(CH3COO)2 + H2C2O4
CaC2O4(к) + 2CH3COOH ⇄ Ca2+ +2 CH3COOЦ + H2C2O4
3. «апишем общую константу совмещенного равновеси€ дл€ реакции исход€ из определени€. ”чтем, что уксусна€ кислота в уравнение входит с коэффициентом 2. «начит, ее константу нужно возвести в квадрат
равн= ѕ–(CaC2O4)∙ 2(CH3COOH) / K1(H2C2O4) ∙ K2(H2C2O4) =
2,3Ј10Ц9∙(1,74 ∙ 10-5)2 / 5,4 ∙10-2 ∙ 5,4 ∙10-5=2,4 ∙ 10-13
Kравн < 1 и K равн < 1Ј10Ц5 оксалат кальци€ в уксусной кислоте не раствор€етс€, равновесие невозможно протекает только обратна€ реакци€.
¬ примерах 1 и 2 совмещаютс€ протолитические и гетерогенные равновеси€; объектом конкуренции €вл€етс€ оксалат-ион C2O42Ц; конкурирующие частицы ионы кальци€ Ca2+ и H+
“≈—“ќ¬џ≈ «јƒјЌ»я “≈ћ≈ VII
ѕ–ќ»«¬≈ƒ≈Ќ»≈ –ј—“¬ќ–»ћќ—“» (ѕ–)
¬ыберите один правильный ответ
1. гетерогенное равновесие трудно растворимой соли ¬а3(–ќ4)2 характеризует уравнение
1) 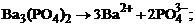
2) 
3) 
4) 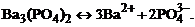
2. теори€ ѕ– не применима ƒл€ соединени€
1) AgJ † 2) AgF†† ††3) AgCl†††† 4) AgBr
3. ѕ– ј1(ќЌ)3 рассчитываетс€ по уравнению:
1) ѕ– = [Al3+]×[OH-]3
2) ѕ– = [Al3+]×3[OH-]
3) ѕ– = [Al]3+ + 3[OH]-†
4) ѕ– = [Al3+] + [OH-]3
4. теори€ ѕ– справедлива дл€
1) ¬а(ќЌ)2 2) Ba(NO3)2 3) BaCl2†††† 4) BaSO4
5. ѕ– соли Hg3(PO4)2 можно рассчитать по выражению
1)  † ††††††††††
† ††††††††††
2) 
3)  †††††††††
†††††††††
4) 
6. образование осадка —aSO4 возможно при значении ѕ
1) 6×10-7†† 2) 5×10-3†††† 3) 5,5×10-8 4) 3,2×10-6
7. –аствор, в котором ѕ < ѕ– €вл€етс€:
|
|
|
1) насыщенны솆††††††††††††
2) ненасыщенным
3) пересыщенны솆††††††††††††††††††††
4) концентрированным
8. –аствор иодида свинца €вл€етс€ при

1) насыщенны솆††††††††††††
2) ненасыщенным
3) пересыщенны솆††††††††††††††††††††
4) концентрированным
9. ѕ раствора фосфата свинца имеет значение, если
††††† 
1) 10-16 2) 10-30†††† 3) 10-25†††† 4) 10-39
10. —мешаны равные объемы  0,001 моль/л раствора и 0,02 моль/л
0,001 моль/л раствора и 0,02 моль/л  раствора. значение ѕ равно
раствора. значение ѕ равно
1)  ††††2)
††††2) 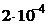 ††3)
††3)  4)
4) 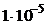
11. ѕ труднорастворимой соли AgCl при смеше-нии 10 мл 0,01 моль/л раствора  †и 5 мл 0,1 моль/л раствора —l равно
†и 5 мл 0,1 моль/л раствора —l равно
1)  †††††2)
†††††2)  †††††††3)
†††††††3)  ††† 4)
††† 4) 
12. концентраци€ ионов  (моль-ион/л) в насы-щенном водном растворе соли
(моль-ион/л) в насы-щенном водном растворе соли  равна†††
равна††† 
1)  †††† 2)
†††† 2)  3)
3)  4)
4) 
13. онцентраци€ ионов  †в насыщенном растворе FeS равна
†в насыщенном растворе FeS равна  . «наче-ние ѕ–
. «наче-ние ѕ–
1)  †††† 2)
†††† 2)  3)
3)  4)
4) 
14. концентраци€ ионов —а2+ выше над осадком
1) CaF2††††††† 2) CaC2O4††††† 3) CaCO3† 4) CaSO4
15. растворимость CdS можно рассчитать по выражению
1)  2)
2)  †††††††
†††††††
3) 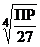 4)
4) 
16. менее растворимо соединение
1) 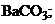 †††††2)
†††††2) 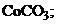 ††††††3)
††††††3)  4)
4) 
17. растворимость  †в воде составл€ет ††
†в воде составл€ет ††  «начение ѕ– сульфида серебра равно
«начение ѕ– сульфида серебра равно
1) 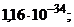 †††2)
†††2)  †††††3)
†††††3)  ††††††4)
††††††4) 
18. величина ѕ– гидроксида хрома (iii), выра-женна€ через растворимость S (моль/л) этого гидроксида
1)  2)
2)  ††††† 3)
††††† 3) 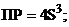 †††††† 4)
†††††† 4) 
19. растворение осадка фосфата кальци€ возможно в кислоте
1)  ††2)
††2)  ††3)
††3)  4) Ќ—ќќЌ
4) Ќ—ќќЌ
20. растворение 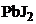 произойдет при добавлении
произойдет при добавлении
1) NaCl 2)  †††3) NaJ††††††† †4)
†††3) NaJ††††††† †4) 
21. растворение осадка  не произойдет при добавлении
не произойдет при добавлении
1)  † 2)
† 2)  †††††
†††††
3) 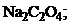 4) KF.
4) KF.
22. ќсадок гидроксида никел€  †раство-ритс€ при добавлении раствора
†раство-ритс€ при добавлении раствора
1) NaOH††††† †††††††††††††††† 2) 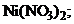 ††††††††††††
††††††††††††
3) HCl †††††† †††††† 4) 
23. Ѕудет ли осадок иодида серебра раствор€тьс€ в уксусной кислоте
1) Ќет, р>1††††††††††† 2) Ќет, р <1†
3) ƒа, р<1†††††††††††††† 4) ƒа, р>1
ќтветы к тесту на стр. 238






