раткие теоретические сведени€
ƒл€ любого труднорастворимого электролита (Kt) х (јn) у между его осадком и насыщенным раствором устанавливаетс€ равновесие вида
(Kt) х (јn) у Û x × Kt z + †+ y × јn z Ц.
†††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††† осадоꆆ††††††††††††††††† насыщенный раствор
онстанта равновеси€ процесса диссоциации мало растворимого вещества в его насыщенном растворе называетс€ произведением растворимости ѕ–.
¬ыражение дл€ произведени€ растворимости имеет вид
ѕ– = 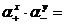 (g + Ј[Kt z + ]) x × (gЦ Ј[јn z Ц ]) y,†††††††††††† (46)
(g + Ј[Kt z + ]) x × (gЦ Ј[јn z Ц ]) y,†††††††††††† (46)
где а +, а Ц Ц активности катиона и аниона; g + , gЦ Ц коэффициенты активностей катиона и аниона; [Kt z + ], [јn z Ц ] Ц равновесные мол€рные концентрации ионов, моль/дм3.
†ѕоскольку насыщенный раствор труднорастворимого вещества содержит небольшие количества ионов, то есть €вл€етс€ достаточно разбавленным, то при расчете ѕ– активности ионов можно заменить равновесными концентраци€ми. “огда уравнение (46) примет вид
ѕ– = [ Kt z + ] x × [јn z Ц ] y. ††††††††††††††††††††††††††††† (47)
ѕ– электролита при данной температуре есть величина посто€нна€. „исленные значени€ произведени€ растворимости большинства труднорастворимых электролитов приведены в справочной литературе.
ѕо величине ѕ– суд€т о растворимости электролита: из двух однотипных соединений большей растворимостью обладает то, произведение растворимости †которого больше.
≈сли произведение концентраций ионов (ѕ–расч) в каком-либо растворе соли больше, чем табличное значение ѕ–табл, то в растворе будет присутствовать осадок данного вещества. » наоборот, если ѕ–расч < ѕ–табл, то осадок данного вещества растворитс€.
–авновесные мол€рные концентрации ионов [Kt z + ]и [јn z Ц ] пропорциональны растворимости вещества L (моль/дм3)
[Kt z + ] = x × L;††††† [јn z Ц ] = y × L. †††††††††††††††††††† (48)
ќтсюда ††††††††††††††††††††ѕ–[(Kt) х (јn) у ] = (x × L) x × (y × L) y; †††††††††††††††††††††††††††††††††††† (49)
L = 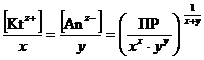 . ††††††††††††††††††††††††† (50)
. ††††††††††††††††††††††††† (50)
ѕримеры решени€ задач
ѕример 1. ¬ычислите произведение растворимости фторида кальци€, если его растворимость в воде равна 0,024 моль/дм3.
– е ш е н и е
”равнение диссоциации имеет вид —аF2↓ Û † —а2+ + 2F Ц. “огда по уравнению (48) равновесные концентрации ионов равны
[—а2+]= L; †[ F Ц]= 2× L.
ѕо формуле (47)
ѕ–(—аF2) = [—а2+]∙[ F Ц]2 = L ×(2 L)2 = 4× L 3 = 4 × (0,024)3 = 5,53 × 10-5.
ѕример 2. ¬ыпадет ли осадок йодида серебра при 25 ∞— после сливани€ 0,1 дм3 0,005 ћ раствора нитрата серебра и 0,3 дм3 0,002 ћ раствора иодида кали€, если ѕ–(AgI)табл = 1,1×10-16?
–ешение
ѕри сливании указанных реактивов идет реакци€
AgNO3 + KI = AgI↓ + KNO3,
Ag+ + IЦ = AgI↓.
ћол€рные концентрации ионов в растворах до смешивани€ равны
[Ag+] = 0,005 моль/дм3; [IЦ] = 0,002 моль/дм3.
|
|
|
онцентрации ионов в растворах после смешивани€
[Ag+] =  =
=  = 1,25∙10-3 моль/дм3;†
= 1,25∙10-3 моль/дм3;†
[IЦ] =  =
=  = 1,5∙10-3 моль/дм3 .
= 1,5∙10-3 моль/дм3 .
ѕо формуле (47) находим произведение концентраций ионов (ѕ–расч)
ѕ–(AgI)расч = [Ag+]×[IЦ]= 1,25∙10-3 ∙1,5∙10-3 = 1,88×10-6.
AgIосаждаетс€, так как соблюдаетс€ условие выпадени€ осадка
ѕ–(AgI)расч > ѕ–(AgI)табл.
ѕример 3. ¬ычислите (не учитыва€ гидролиза) растворимость фосфата бари€ в моль/л и г/л, а также мол€рные концентрации ионов в насыщенном растворе данной соли, если ѕ– [¬а3(–ќ4)2] = 6,3 Ј 10-39.
– е ш е н и е
‘осфат бари€ диссоциирует по схеме ¬а3(–ќ4)2↓↔ 3¬а2+ + 2–ќ  , тогда по формуле (47)
, тогда по формуле (47)
ѕ– [¬а3(–ќ4)2] †= [¬а2+]3 Ј [–ќ  ]2.
]2.
ѕо уравнению (48) равновесные концентрации ионов равны
[¬а2+] = 3× L;†††††† [–ќ  ] = 2× L.
] = 2× L.
“огда по по формуле (50) †† L = 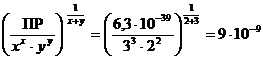 †моль/л.
†моль/л.
ћол€рна€ масса фосфата бари€ ћ 2(¬а3(–ќ4)2) = 602 г/моль, тогда растворимость, выраженна€ в г/л
L = 602 Ј 9 Ј 10-9 = 5,42 Ј 10-6 г/л.
онцентрации ионов [¬а2+] = 3× L = 3 Ј 9 Ј 10-9 = 2,7 Ј 10-8 моль/л;
[–ќ  ] = 2× L = 9 Ј 10-9 Ј 2 = 1,8 Ј 10-8 моль/л.
] = 2× L = 9 Ј 10-9 Ј 2 = 1,8 Ј 10-8 моль/л.
ѕример 4. ¬ыпадет ли осадок галогенида серебра, если к 1 л †0,1 ћ раствора [Ag(NH3)2]NO3, содержащему 1 моль аммиака добавить:
а) 1×10Ц5 моль ¬r; †††††††б) 1×10Ц5 моль I.
– е ш е н и е
ƒл€ решени€ вопроса о возможности разрушени€ комплексного иона за счет св€зывани€ комплексообразовател€ в малорастворимую соль необходимо оценить значени€ равновесных концентраций ионов в рассматриваемой системе. ƒл€ этого из справочника [8] выбираем значение константы нестойкости комплекса и произведени€ растворимости соответствующих солей
Ќ([Ag(NH3)2]+) = 5,9 Ј 10Ц8; †ѕ–(AgBr) = 5,3 Ј 10Ц13; ѕ–(AgI) = 8,3 Ј 10Ц17.
††† ƒанна€ комплексна€ соль диссоциирует по схеме
[Ag(NH3)2]NO3 Ѓ [Ag(NH3)2]+ + NO  .
.
ѕо определению дл€ комплексного иона [Ag(NH3)2]+
Ќ([Ag(NH3)2]+) =  .
.
[NH3] ≈ —2 (NH3) = 1 моль/л, так как концентраци€ молекул аммиака, образовавшихс€ вследствие вторичной диссоциации комплексного иона мала.
»з схемы первичной диссоциации следует, что
[[Ag(NH3)2]+] ≈ — 2([Ag(NH3)2]NO3) = 0,1 моль/л.
“огда концентраци€ ионов Ag+ равна
[Ag+] =  моль/л.
моль/л.
онцентрацию ионов Br Ц, достаточную дл€ осаждени€ AgBr найдем из выражени€ дл€ ѕ– (AgBr)
[Br Ц] =  †моль/л.
†моль/л.
“ак как необходима€ дл€ осаждени€ бромида серебра концентраци€ ионов брома ([Br Ц] = 8,98×10Ц5 моль/дм3) больше добавл€емой в составе бромида кали€ ([Br Ц] = 1×10Ц5 моль/дм3), то осадок бромида серебра не выпадает.
онцентрацию ионов IЦ, достаточную дл€ осаждени€ AgI найдем по аналогии
[IЦ] =  моль/л.
моль/л.
“ак как необходима€ дл€ осаждени€ йодида серебра концентраци€ ионов йода ([I Ц] = 1,41×10Ц8 моль/дм3) меньше добавл€емой в составе йодида кали€ ([I Ц] = 1×10Ц5 моль/дм3), то реакци€ разрушени€ комплексного иона в данном случае будет протекать
[Ag(NH3)2]NO3 + 2 I = AgI↓ + NO3 + 2NH3.
√»ƒ–ќЋ»« —ќЋ≈…






