—ера Ц элемент 3‑го периода и VIA‑группы ѕериодической системы, пор€дковый номер 16, относитс€ к халькогенам. Ёлектронна€ формула атома [10Ne]3s23p4, характерные степени окислени€ 0, ‑II, +IV и +VI, состо€ние SVI считаетс€ устойчивым.
Ўкала степеней окислени€ серы:

Ёлектроотрицательность серы равна 2,60, дл€ нее характерны неметаллические свойства. ¬ водородных и кислородных соединени€х находитс€ в составе различных анионов, образует кислородсодержащие кислоты и их соли, бинарные соединени€.
¬ природе Ц п€тнадцатый по химической распространенности элемент (седьмой среди неметаллов). ¬стречаетс€ в свободном (самородном) и св€занном виде. ∆изненно важный элемент дл€ высших организмов.
—ера S. ѕростое вещество. ∆елта€ кристаллическа€ (α‑ромбическа€ и β‑моноклинна€, 
при 95,5 ∞C) или аморфна€ (пластическа€). ¬ узлах кристаллической решетки наход€тс€ молекулы S8 (неплоские циклы типа Ђкоронаї), аморфна€ сера состоит из цепей Sn. Ќизкоплавкое вещество, в€зкость жидкости проходит через максимум при 200 ∞C (разрыв молекул S8, переплетение цепей Sn). ¬ паре Ц молекулы S8, S6, S4, S2. ѕри 1500 ∞C по€вл€етс€ одноатомна€ сера (в химических уравнени€х дл€ простоты люба€ сера изображаетс€ как S).
—ера не раствор€етс€ в воде и при обычных услови€х не реагирует с ней, хорошо растворима в сероуглероде CS2.
—ера, особенно порошкообразна€, обладает высокой активностью при нагревании. –еагирует как окислитель с металлами и неметаллами:

а как восстановитель Ц с фтором, кислородом и кислотами (при кип€чении):

—ера подвергаетс€ дисмутации в растворах щелочей:
3S0 + 6 ќЌ (конц.) = 2K2S‑II + K2SIVO3 + 3H2O
ѕри высокой температуре (400 ∞C) сера вытесн€ет иод из иодоводорода:
S + 2ЌI(г) = I2 + H2S,
но в растворе реакци€ идет в обратную сторону:
I2 + H2S(p) = 2 HI + S↓
ѕолучение: в промышленности выплавл€етс€ из природных залежей самородной серы (с помощью вод€ного пара), выдел€етс€ при десульфурации продуктов газификации угл€.
—ера примен€етс€ дл€ синтеза сероуглерода, серной кислоты, сернистых (кубовых) красителей, при вулканизации каучука, как средство защиты растений от мучнистой росы, дл€ лечени€ кожных заболеваний.
—ероводород H2S. Ѕескислородна€ кислота. Ѕесцветный газ с удушающим запахом, т€желее воздуха. ћолекула имеет строение дважды незавершенного тетраэдра [::S(H)2]
(sp3‑гибридизаци€, валетный угол Ќ Ц SЦЌ далек от тетраэдрического). Ќеустойчив при нагревании выше 400 ∞C. ћалорастворим в воде (2,6 л/1 л Ќ2O при 20 ∞C), насыщенный раствор децимол€рный (0,1ћ, Ђсероводородна€ водаї). ќчень слаба€ кислота в растворе, практически не диссоциирует по второй стадии до ионов S2‑ (максимальна€ концентраци€ S2‑ равна 1 10‑13 моль/л). ѕри сто€нии на воздухе раствор мутнеет (ингибитор Ц сахароза). Ќейтрализуетс€ щелочами, не полностью Ц гидратом аммиака. —ильный восстановитель. ¬ступает в реакции ионного обмена. —ульфидирующий агент, осаждает из раствора разноокрашенные сульфиды с очень малой растворимостью.
|
|
|
ачественные реакции Ц осаждение сульфидов, а также неполное сгорание H2S с образованием желтого налета серы на внесенном в плам€ холодном предмете (фарфоровый шпатель). ѕобочный продукт очистки нефти, природного и коксового газа.
ѕримен€етс€ в производстве серы, неорганических и органических серосодержащих соединений как аналитический реагент. „резвычайно €довит. ”равнени€ важнейших реакций:


ѕолучение: в промышленности Ц пр€мым синтезом:
Ќ2 + S = H2S (150Ц200 ∞C)
или при нагревании серы с парафином;
в лаборатории Ц вытеснением из сульфидов сильными кислотами
FeS + 2ЌCl (конц.) = FeCl2 + H2S↑
или полным гидролизом бинарных соединений:
Al2S3 + 6Ќ2O = 2Al(ќЌ)3↓ + 3 H2S↑
—ульфид натри€ Na2S. Ѕескислородна€ соль. Ѕелый, очень гигроскопичный. ѕлавитс€ без разложени€, термически устойчивый. ’орошо растворим в воде, гидролизуетс€ по аниону, создает в растворе сильнощелочную среду. ѕри сто€нии на воздухе раствор мутнеет (коллоидна€ сера) и желтеет (окраска полисульфида). “ипичный восстановитель. ѕрисоедин€ет серу. ¬ступает в реакции ионного обмена.
ачественные реакции на ион S2‑ Ц осаждение разноокрашенных сульфидов металлов, из которых MnS, FeS, ZnS разлагаютс€ в ЌCl (разб.).
ѕримен€етс€ в производстве сернистых красителей и целлюлозы, дл€ удалени€ волос€ного покрова шкур при дублении кож, как реагент в аналитической химии.
”равнени€ важнейших реакций:
Na2S + 2ЌCl (разб.) = 2NaCl + H2S↑
Na2S + 3H2SO4 (конц.) = SO2↑ + S↓ + 2H2O + 2NaHSO4 (до 50 ∞C)
Na2S + 4HNO3 (конц.) = 2NO↑ + S↓ + 2H2O + 2NaNO3 (60 ∞C)
Na2S + H2S (насыщ.) = 2NaHS
Na2S(т) + 2O2 = Na2SO4 (выше 400 ∞C)
Na2S + 4H2O2 (конц.) = Na2SO4 + 4H2O
S2‑ + M2+ = MnS (телесн.)↓; FeS (черн.)↓; ZnS (бел.)↓
S2‑ + 2Ag+ = Ag2S (черн.)↓
S2‑ + M2+ = —dS (желт.)↓; PbS, CuS, HgS (черные)↓
3S2‑ + 2Bi3+ = Bi2S3 (кор. Ц черн.)↓
3S2‑ + 6H2O + 2M3+ = 3H2S↑ + 2M(OH)3↓ (M = Al, Cr)
ѕолучение в промышленности Ц прокаливание минерала мирабилит Na2SO4 10Ќ2O в присутствии восстановителей:
Na2SO4 + 4Ќ2 = Na2S + 4Ќ2O (500 ∞C, кат. Fe2O3)
Na2SO4 + 4— (кокс) = Na2S + 4—ќ (800Ц1000 ∞C)
Na2SO4 + 4—ќ = Na2S + 4—O2 (600Ц700 ∞C)
—ульфид алюмини€ Al2S3. Ѕескислородна€ соль. Ѕелый, св€зь Al Ц S преимущественно ковалентна€. ѕлавитс€ без разложени€ под избыточным давлением N2, легко возгон€етс€. ќкисл€етс€ на воздухе при прокаливании. ѕолностью гидролизуетс€ водой, не осаждаетс€ из раствора. –азлагаетс€ сильными кислотами. ѕримен€етс€ как твердый источник чистого сероводорода. ”равнени€ важнейших реакций:
Al2S3 + 6Ќ2O = 2Al(ќЌ)3↓ + 3H2S↑ (чистый)
Al2S3 + 6ЌCl (разб.) = 2AlCl3 + 3H2S↑
Al2S3 + 24HNO3 (конц.) = Al2(SO4)3 + 24NO2↑ + 12H2O (100 ∞C)
2Al2S3 + 9O2 (воздух) = 2Al2O3 + 6SO2 (700Ц800 ∞C)
ѕолучение: взаимодействие алюмини€ с расплавленной серой в отсутствие кислорода и влаги:
2Al + 3S = AL2S3 (150Ц200 ∞C)
—ульфид железа (II) FeS. Ѕескислородна€ соль. „ерно‑серый с зеленым оттенком, тугоплавкий, разлагаетс€ при нагревании в вакууме. ¬о влажном состо€нии чувствителен к кислороду воздуха. Ќерастворим в воде. Ќе выпадает в осадок при насыщении растворов солей железа(II) сероводородом. –азлагаетс€ кислотами. ѕримен€етс€ как сырье в производстве чугуна, твердый источник сероводорода.
|
|
|
—оединение железа(III) состава Fe2S3 не известно (не получено).
”равнени€ важнейших реакций:

ѕолучение:
Fe + S = FeS (600 ∞C)
Fe2O3 + H2 + 2H2S = 9 FeS + 3H2O (700‑1000 ∞C)
FeCl2 + 2NH4HS (изб.) = FeS ↓ + 2NH4Cl + H2S↑
ƒисульфид железа FeS2. Ѕинарное соединение. »меет ионное строение Fe2+ (ЦS Ц SЦ)2‑. “емно‑желтый, термически устойчивый, при прокаливании разлагаетс€. Ќерастворим в воде, не реагирует с разбавленными кислотами, щелочами. –азлагаетс€ кислотами‑окислител€ми, подвергаетс€ обжигу на воздухе. ѕримен€етс€ как сырье в производстве чугуна, серы и серной кислоты, катализатор в органическом синтезе. ¬ природе Ц рудные минералы пирит и марказит.
”равнени€ важнейших реакций:
FeS2 = FeS + S (выше 1170 ∞C, вакуум)
2FeS2 + 14H2SO4 (конц., гор.) = Fe2(SO4)3 + 15SO2↑ + 14Ќ2O
FeS2 + 18HNO3 (конц.) = Fe(NO3)3 + 2H2SO4 + 15NO2↑ + 7H2O
4FeS2 + 11O2 (воздух) = 8SO2 + 2Fe2O3 (800 ∞C, обжиг)
√идросульфид аммони€ NH4HS. Ѕескислородна€ кисла€ соль. Ѕелый, плавитс€ под избыточным давлением. ¬есьма летучий, термически неустойчивый. Ќа воздухе окисл€етс€. ’орошо растворим в воде, гидролизуетс€ по катиону и аниону (преобладает), создает щелочную среду. –аствор желтеет на воздухе. –азлагаетс€ кислотами, в насыщенном растворе присоедин€ет серу. ўелочами не нейтрализуетс€, средн€€ соль (NH4)2S не существует в растворе (услови€ получени€ средней соли см. в рубрике ЂH2Sї). ѕримен€етс€ в качестве компонента фотопро€вителей, как аналитический реагент (осадитель сульфидов).
”равнени€ важнейших реакций:
NH4HS = NH3 + H2S (выше 20 ∞C)
NH4HS + ЌCl (разб.) = NH4Cl + H2S↑
NH4HS + 3HNO3 (конц.) = S↓ + 2NO2↑ + NH4NO3 + 2H2O
2NH4HS (насыщ. H2S) + 2CuSO4 = (NH4)2SO4 + H2SO4 + 2CuS↓
ѕолучение: насыщение концентрированного раствора NH3 сероводородом:
NH3 Ќ2O (конц.) + H2S(г) = NH4HS + Ќ2O
¬ аналитической химии раствор, содержащий равные количества NH4HS и NH3 Ќ2O, условно считают раствором (NH4)2S и используют формулу средней соли в записи уравнений реакций, хот€ сульфид аммони€ полностью гидролизуетс€ в воде до NH4HS и NH3 Х Ќ2O.
ƒиоксид серы. —ульфиты
ƒиоксид серы SO2. ислотный оксид. Ѕесцветный газ с резким запахом. ћолекула имеет строение незавершенного треугольника [: S(O)2] (sр2‑гибридизаци€), содержит σ,π‑св€зи S=O. Ћегко сжижаетс€, термически устойчивый. ’орошо растворим в воде (~40 л/1 л Ќ2O при 20 ∞C). ќбразует полигидрат, обладающий свойствами слабой кислоты, продукты диссоциации Ц ионы HSO3‑ и SO32‑. »он HSO3‑ имеет две таутомерные формы Ц симметричную (некислотную) со строением тетраэдра [S(H)(O)3] (sр3‑гибридизаци€), котора€ преобладает в смеси, и несимметричную (кислотную) со строением незавершенного тетраэдра [: S(O)2(OH)] (sр3‑гибридизаци€). »он SO32‑ также тетраэдрический [: S(O)3].
–еагирует со щелочами, гидратом аммиака. “ипичный восстановитель, слабый окислитель.
ачественна€ реакци€ Ц обесцвечивание желто‑коричневой Ђйодной водыї. ѕромежуточный продукт в производстве сульфитов и серной кислоты.
ѕримен€етс€ дл€ отбеливани€ шерсти, шелка и соломы, консервировани€ и хранени€ фруктов, как дезинфицирующее средство, антиоксидант, хладагент. ядовит.
—оединение состава H2SO3 (серниста€ кислота) не известно (не существует).
”равнени€ важнейших реакций:


–астворение в воде и кислотные свойства:

ѕолучение: в промышленности Ц сжигание серы в воздухе, обогащенном кислородом, и, в меньшей степени, обжиг сульфидных руд (SO2 Ц попутный газ при обжиге пирита):
S + O2 = SO2 (280Ц360 ∞C)
4FeS2 + 11O2 = 2Fe2O3 + 8 SO2 (800 ∞C, обжиг)
в лаборатории Ц вытеснение серной кислотой из сульфитов:
|
|
|
BaSO3(т) + H2SO4 (конц.) = BaSO4↓ + SO2↑ + Ќ2O
—ульфит натри€ Na2SO3. ќксосоль. Ѕелый. ѕри нагревании на воздухе разлагаетс€ без плавлени€, плавитс€ под избыточным давлением аргона. ¬о влажном состо€нии и в растворе чувствителен к кислороду воздуха. ’орошо растворим в воде, гидролизуетс€ по аниону. –азлагаетс€ кислотами. “ипичный восстановитель.
ачественна€ реакци€ на ион SO32‑ Ц образование белого осадка сульфита бари€, который переводитс€ в раствор сильными кислотами (ЌCl, HNO3).
ѕримен€етс€ как реактив в аналитической химии, компонент фотографических растворов, нейтрализатор хлора при отбеливании тканей.
”равнени€ важнейших реакций:

ѕолучение:
Na2CO3 (конц.) + SO2 = Na2SO3 + CO2↑
—ерна€ кислота. —ульфаты
—ерна€ кислота H2SO4. ќксокислота. Ѕесцветна€ жидкость, очень в€зка€ (маслообразна€), весьма гигроскопична€. ћолекула имеет искаженно‑тетраэдрическое строение [S(O)2(OH)2] (sр3‑гибридизаци€), содержит ковалентные σ‑св€зи S Ц ќЌ и σπ‑св€зи S=O. »он SO42‑ имеет правильно‑тетраэдрическое строение [S(O)4]. ќбладает широким температурным интервалом жидкого состо€ни€ (~300 градусов). ѕри нагревании выше 296 ∞C частично разлагаетс€. ѕерегон€етс€ в виде азеотропной смеси с водой (массова€ дол€ кислоты 98,3 %, температура кипени€ 296Ц340 ∞C), при более сильном нагревании разлагаетс€ полностью. Ќеограниченно смешиваетс€ с водой (с сильным экзо ‑эффектом). —ильна€ кислота в растворе, нейтрализуетс€ щелочами и гидратом аммиака. ѕереводит металлы в сульфаты (при избытке концентрированной кислоты в обычных услови€х образуютс€ растворимые гидросульфаты), но металлы Be, Bi, —о, Fe, Mg и Nb пассивируютс€ в концентрированной кислоте и не реагируют с ней. –еагирует с основными оксидами и гидроксидами, разлагает соли слабых кислот. —лабый окислитель в разбавленном растворе (за счет ЌI), сильный Ц в концентрированном растворе (за счет SVI). ’орошо раствор€ет SO3 и реагирует с ним (образуетс€ т€жела€ маслообразна€ жидкость Ц олеум, содержит H2S2O7).
ачественна€ реакци€ на ион SO42‑ Ц осаждение белого сульфата бари€ BaSO4 (осадок не переводитс€ в раствор сол€ной и азотной кислотами, в отличие от белого осадка BaSO3).
ѕримен€етс€ в производстве сульфатов и других соединений серы, минеральных удобрений, взрывчатых веществ, красителей и лекарственных препаратов, в органическом синтезе, дл€ Ђвскрыти€ї (первого этапа переработки) промышленно важных руд и минералов, при очистке нефтепродуктов, электролизе воды, как электролит свинцовых аккумул€торов. ядовита, вызывает ожоги кожи. ”равнени€ важнейших реакций:

ѕолучение в промышленности:
а) синтез SO2 из серы, сульфидных руд, сероводорода и сульфатных руд:
S + O2 (воздух) = SO2 (280Ц360 ∞C)
4FeS2 + 11O2 (воздух) = 8 SO2 + 2Fe2O3 (800 ∞C, обжиг)
2H2S + 3O2 (изб.) = 2 SO2 + 2Ќ2O (250Ц300 ∞C)
CaSO4 + — (кокс) = —аќ + SO2 + —ќ (1300Ц1500 ∞C)
б) конверси€ SO2 в SO3 в контактном аппарате:

в) синтез концентрированной и безводной серной кислоты:
Ќ2O (разб. H2SO4) + SO3 = H2SO4 (конц., безводн.)
(поглощение SO3 чистой водой с получением H2SO4 не проводитс€ из‑за сильного разогревани€ смеси и обратного разложени€ H2SO4, см. выше);
г) синтез олеума Ц смеси безводной H2SO4, дисерной кислоты H2S2O7 и избыточного SO3. –астворенный SO3 гарантирует безводность олеума (при попадании воды тут же образуетс€ H2SO4), что позвол€ет безопасно перевозить его в стальных цистернах.
—ульфат натри€ Na2SO4. ќксосоль. Ѕелый, гигроскопичный. ѕлавитс€ и кипит без разложени€. ќбразует кристаллогидрат (минерал мирабилит), легко тер€ющий воду; техническое название глауберова соль. ’орошо растворим в воде, не гидролизуетс€. –еагирует с H2SO4 (конц.), SO3. ¬осстанавливаетс€ водородом, коксом при нагревании. ¬ступает в реакции ионного обмена.
|
|
|
ѕримен€етс€ в производстве стекла, целлюлозы и минеральных красок, как лекарственное средство. —одержитс€ в рапе сол€ных озер, в частности в заливе ара‑Ѕогаз‑√ол аспийского мор€.
”равнени€ важнейших реакций:
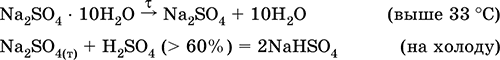

√идросульфат кали€ KHSO4. исла€ оксосоль. Ѕелый, гигроскопичный, но кристаллогидратов не образует. ѕри нагревании плавитс€ и разлагаетс€. ’орошо растворим в воде, в растворе анион подвергаетс€ диссоциации, среда раствора сильнокислотна€. Ќейтрализуетс€ щелочами.
ѕримен€етс€ как компонент флюсов в металлургии, составна€ часть минеральных удобрений.
”равнени€ важнейших реакций:
2KHSO4 = K2SO4 + H2SO4 (до 240 ∞C)
2KHSO4 = K2S2O7 + Ќ2O (320Ц340 ∞C)
KHSO4 (разб.) + ќЌ (конц.) = K2SO4 + Ќ2O KHSO4 + Cl = K2SO4 + ЌCl (450Ц700 ∞C)
6KHSO4 + ћ2O3 = 2KM(SO4)2 + 2K2SO4 + 3H2O (350Ц500 ∞C, M = Al, Cr)
ѕолучение: обработка сульфата кали€ концентрированной (более чем 6O%‑ной) серной кислотой на холоду:
K2SO4 + H2SO4 (конц.) = 2 KHSO4
—ульфат кальци€ CaSO4. ќксосоль. Ѕелый, весьма гигроскопичный, тугоплавкий, при прокаливании разлагаетс€. ѕриродный CaSO4 встречаетс€ в виде очень распространенного минерала гипс CaSO4 2Ќ2O. ѕри 130 ∞C гипс тер€ет часть воды и переходит в жжЄный (штукатурный) гипс 2CaSO4 Х Ќ2O (техническое название алебастр). ѕолностью обезвоженный (200 ∞C) гипс отвечает минералу ангидрит CaSO4. ћалорастворим в воде (0,206 г/100 г Ќ2O при 20 ∞C), растворимость уменьшаетс€ при нагревании. –еагирует с H2SO4 (конц.). ¬осстанавливаетс€ коксом при сплавлении. ќпредел€ет большую часть Ђпосто€ннойї жесткости пресной воды (подробнее см. 9.2).
”равнени€ важнейших реакций: 100Ц128 ∞C

ѕримен€етс€ как сырье в производстве SO2, H2SO4 и (NH4)2SO4, как флюс в металлургии, наполнитель бумаги. ѕриготовленный из жженого гипса в€жущий строительный раствор Ђсхватываетс€ї быстрее, чем смесь на основе —а(ќЌ)2. «атвердевание обеспечиваетс€ св€зыванием воды, образованием гипса в виде каменной массы. »спользуетс€ жженый гипс дл€ изготовлени€ гипсовых слепков, архитектурно‑декоративных форм и изделий, перегородочных плит и панелей, каменных полов.
—ульфат алюмини€‑кали€ KAl(SO4)2. ƒвойна€ оксосоль. Ѕелый, гигроскопичный. ѕри сильном нагревании разлагаетс€. ќбразует кристаллогидрат Ц алюжокалиевые квасцы. ”меренно растворим в воде, гидролизуетс€ по катиону алюмини€. –еагирует со щелочами, гидратом аммиака.
ѕримен€етс€ как протрава при крашении тканей, дубитель кож, коагул€нт при очистке пресной воды, компонент составов дл€ проклеивани€ бумаги, наружное кровоостанавливающее средство в медицине и косметологии. ќбразуетс€ при совместной кристаллизации сульфатов алюмини€ и кали€.
”равнени€ важнейших реакций:


—ульфат хрома(III) Ц кали€ KCr(SO4)2. ƒвойна€ оксосоль. расный (гидрат темно‑фиолетовый, техническое название хрожокалиевые квасцы). ѕри нагревании разлагаетс€ без плавлени€. ’орошо растворим в воде (серо‑син€€ окраска раствора отвечает аквакомплексу [Cr(Ќ2O)6]3+), гидролизуетс€ по катиону хрома(III). –еагирует со щелочами, гидратом аммиака. —лабый окислитель и восстановитель. ¬ступает в реакции ионного обмена.
ачественные реакции на ион Cr3+ Ц восстановление до Cr2+ или окисление до желтого CrO42‑.
ѕримен€етс€ как дубитель кож, протрава при крашении тканей, реактив в фотографии. ќбразуетс€ при совместной кристаллизации сульфатов хрома(III) и кали€. ”равнени€ важнейших реакций:


—ульфат марганца (II) MnSO4. ќксосоль. Ѕелый, при прокаливании плавитс€ и разлагаетс€. ристаллогидрат MnSO4 5Ќ2O Ц красно‑розовый, техническое название марганцевый купорос. ’орошо растворим в воде, светло‑розова€ (почти бесцветна€) окраска раствора отвечает аквакомплексу [Mn(Ќ2O)6]2+; гидролизуетс€ по катиону. –еагирует со щелочами, гидратом аммиака. —лабый восстановитель, реагирует с типичными (сильными) окислител€ми.
ачественные реакции на ион Mn2+ Ц конмутаци€ с ионом MnO4 и исчезновение фиолетовой окраски последнего, окисление Mn2+ до MnO4 и по€вление фиолетовой окраски.
ѕримен€етс€ дл€ получени€ Mn, MnO2 и других соединений марганца, как микроудобрение и аналитический реагент.
”равнени€ важнейших реакций:

ѕолучение:
2MnO2 + 2H2SO4 (конц.) = 2 MnSO4 + O2↑ + 2H2O (100 ∞C)
|
|
|
—ульфат железа (II) FeSO4. ќксосоль. Ѕелый (гидрат светло‑зеленый, техническое название железный купорос), гигроскопичный. –азлагаетс€ при нагревании. ’орошо растворим в воде, в малой степени гидролизуетс€ по катиону. Ѕыстро окисл€етс€ в растворе кислородом воздуха (раствор желтеет и мутнеет). –еагирует с кислотами‑окислител€ми, щелочами, гидратом аммиака. “ипичный восстановитель.
ѕримен€етс€ как компонент минеральных красок, электролитов в гальванотехнике, консервант древесины, фунгицид, лекарственное средство против анемии. ¬ лаборатории чаще беретс€ в виде двойной соли Fe(NH4)2(SO4)2 6Ќ2O (соль ћора), более устойчивой к действию воздуха.
”равнени€ важнейших реакций:

ѕолучение:
Fe + H2SO4 (разб.) = FeSO4 + H2↑
FeCO3 + H2SO4 (разб.) = FeSO4 + CO2↑ + H2O
7.4. Ќеметаллы VA‑группы
јзот. јммиак
јзот Ц элемент 2‑го периода и VA‑группы ѕериодической системы, пор€дковый номер 7. Ёлектронна€ формула атома [2He]2s22p3, характерные степени окислени€ 0, ‑III, +III и +V, реже +II, +IV и др.; состо€ние Nv считаетс€ относительно устойчивым.
Ўкала степеней окислени€ азота:

јзот обладает высокой электроотрицательностью (3,07), третий после F и ќ. ѕро€вл€ет типичные неметаллические (кислотные) свойства. ќбразует различные кислородсодержащие кислоты, соли и бинарные соединени€, а также катион аммони€ NH4+ и его соли.
¬ природе Ц семнадцатый по химической распространенности элемент (дев€тый среди неметаллов). ∆изненно важный элемент дл€ всех организмов.
јзот N2. ѕростое вещество. —остоит из непол€рных молекул с очень устойчивой σππ‑св€зью N ≡ N, этим объ€сн€етс€ химическа€ инертность азота при обычных услови€х. Ѕесцветный газ без вкуса и запаха, конденсируетс€ в бесцветную жидкость (в отличие от O2).
√лавна€ составна€ часть воздуха: 78,09 % по объему, 75,52 % по массе. »з жидкого воздуха азот выкипает раньше кислорода O2. ћалорастворим в воде (15,4 мл/1 л Ќ2O при 20 ∞C), растворимость азота меньше, чем у кислорода.
ѕри комнатной температуре N2 реагирует только с литием (во влажной атмосфере), образу€ нитрид лити€ Li3N, нитриды других элементов синтезируют при сильном нагревании:
N2 + 3Mg = Mg3N2 (800 ∞C)
¬ электрическом разр€де N2 реагирует с фтором и в очень малой степени Ц с кислородом:

ќбратима€ реакци€ получени€ аммиака протекает при 500 ∞C, под давлением до 350 атм и об€зательно в присутствии катализатора (Fe/F2O3/FeO, в лаборатории Pt):

¬ соответствии с принципом Ће‑Ўателье увеличение выхода аммиака должно происходить при повышении давлени€ и понижении температуры. ќднако скорость реакции при низких температурах очень мала, поэтому процесс ведут при 450Ц500 ∞C, достига€ 15 %‑ного выхода аммиака. Ќепрореагировавшие N2 и Ќ2 возвращают в реактор и тем самым увеличивают степень протекани€ реакции.
јзот химически пассивен по отношению к кислотам и щелочам, не поддерживает горени€.
ѕолучение в промышленности Ц фракционна€ дистилл€ци€ жидкого воздуха или удаление из воздуха кислорода химическим путем, например по реакции 2— (кокс) + O2 = 2—ќ при нагревании. ¬ этих случа€х получают азот, содержащий также примеси благородных газов (главным образом аргон).
¬ лаборатории небольшие количества химически чистого азота можно получить по реакции конмутации при умеренном нагревании:
N‑IIIH4NIIIO2(т) = N20 + 2H2O (60Ц70 ∞C)
NH4Cl(p) + KNO2(p) = N20↑ + KCl + 2H2O (100 ∞C)
ѕримен€етс€ дл€ синтеза аммиака, азотной кислоты и других азотсодержащих продуктов, как инертна€ среда проведени€ химических и металлургических процессов и хранени€ огнеопасных веществ.
јммиак NH3. Ѕинарное соединение, степень окислени€ азота равна Ц III. Ѕесцветный газ с резким характерным запахом. ћолекула имеет строение незавершенного тетраэдра [: N(H)3)] (sр3‑гибридизаци€). Ќаличие у азота в молекуле NH3 донорной пары электронов на sр3‑гибридной орбитали обусловливает характерную реакцию присоединени€ катиона водорода, при этом образуетс€ катион аммони€ NH4+. —жижаетс€ под избыточным давлением при комнатной температуре. ¬ жидком состо€нии ассоциирован за счет водородных св€зей. “ермически неустойчив. ’орошо растворим в воде (более 700 л/1 л Ќ2O при 20 ∞C); дол€ в насыщенном растворе равна = 34 % по массе и = 99 % по объему, рЌ = 11,8.
¬есьма реакционноспособный, склонен к реакци€м присоединени€. Crорает в кислороде, реагирует с кислотами. ѕро€вл€ет восстановительные (за счет N‑III) и окислительные (за счет ЌI) свойства. ќсушаетс€ только оксидом кальци€.
ачественные реакции Ц образование белого Ђдымаї при контакте с газообразным ЌCl, почернение бумажки, смоченной раствором Hg2(NO3)2.
ѕромежуточный продукт при синтезе HNO3 и солей аммони€. ѕримен€етс€ в производстве соды, азотных удобрений, красителей, взрывчатых веществ; жидкий аммиак Ц хладагент. ядовит.
”равнени€ важнейших реакций:

ѕолучение: в лаборатории Ц вытеснение аммиака из солей аммони€ при нагревании с натронной известью (NaOH + —аќ):

или кип€чение водного раствора аммиака с последующим осушением газа.
¬ промышленности аммиак синтезируют из азота (см.) с водородом. ¬ыпускаетс€ промышленностью либо в сжиженном виде, либо в виде концентрированного водного раствора под техническим названием аммиачна€ вода.
√идрат аммиака NH3 Ќ2O. ћежмолекул€рное соединение. Ѕелый, в кристаллической решетке Ц молекулы NH3 и Ќ2O, св€занные слабой водородной св€зью H3NЕ ЌќЌ. ѕрисутствует в водном растворе аммиака, слабое основание (продукты диссоциации Ц катион NH4‑ и анион ќЌ‑). атион аммони€ имеет правильно‑тетраэдрическое строение (sp3‑гибридизаци€). “ермически неустойчив, полностью разлагаетс€ при кип€чении раствора. Ќейтрализуетс€ сильными кислотами. ѕро€вл€ет восстановительные свойства (за счет NIII) в концентрированном растворе. ¬ступает в реакции ионного обмена и комплексообразовани€.
ачественна€ реакци€ Ц образование белого Ђдымаї при контакте с газообразным ЌCl.
ѕримен€етс€ дл€ создани€ слабощелочной среды в растворе, при осаждении амфотерных гидроксидов.
¬ 1ћ растворе аммиака содержитс€ в основном гидрат NH3 Ќ2O и лишь 0,4 % ионов NH4+ и ќЌ‑ (за счет диссоциации гидрата); таким образом, ионный Ђгидроксид аммони€ NH4OHї практически не содержитс€ в растворе, нет такого соединени€ и в твердом гидрате. ”равнени€ важнейших реакций:
NH3 Ќ2O (конц.) = NH3↑ + Ќ2O (кип€чение с NaOH)
NH3 Ќ2O + ЌCl (разб.) = NH4Cl + Ќ2O
3(NH3 Ќ2O) (конц.) + CrCl3 = Cr(OH)3↓ + 3NH4Cl
8(NH3 Ќ2O) (конц.) + «Br2(р) = N2↑ + 6NH4Br + 8Ќ2O (40Ц50 ∞C)
2(NH3 Ќ2O) (конц.) + 2 MnO4 = N2↑ + 2MnO2↓ + 4Ќ2O + 2 ќЌ
4(NH3 Ќ2O) (конц.) + Ag2O = 2[Ag(NH3)2]OH + 3H2O
4(NH3 Ќ2O) (конц.) + Cu(OH)2 + [Cu(NH3)4](OH)2 + 4Ќ2O
6(NH3 Ќ2O) (конц.) + NiCl2 = [Ni(NH3)6]Cl2 + 6Ќ2O
–азбавленный раствор аммиака (3Ц10 %‑ный) часто называют нашатырным спиртом (название придумано алхимиками), а концентрированный раствор (18,5Ц25 %‑ный) Ц аммиачной водой (выпускаетс€ промышленностью).






