ѕр€мое титрование. –ешение типовых задач по результатам пр€мого титровани€ основано на использовании закона эквивалентов:
C ( X) Ј V (X) = C (
X) Ј V (X) = C ( R) Ј V (R).
R) Ј V (R).
ѕример. »з навески 2CO3 массой 1,3811 г приготовили 200,0 мл раствора. Ќа титрование 15,0 мл раствора израсходовали 11,3 мл раствора Ќ2SO4. –ассчитать мол€рную концентрацию раствора Ќ2SO4 и ее титр по цинку “ (Ќ2SO4/Zn).
–ешение. –ассчитаем мол€рную концентрацию эквивалента приготовленного раствора 2CO3 с учетом фактора эквивалентности, равного  :
:
— ( 2CO3) =
2CO3) = 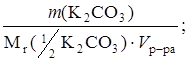
— ( 2CO3) =
2CO3) =  = 0,09993 моль/л.
= 0,09993 моль/л.
»спользу€ закон эквивалентов, определим мол€рную концентрацию эквивалента Ќ2SO4 (f экв =  ):
):
— ( Ќ2SO4) =
Ќ2SO4) =  ;
;
— ( Ќ2SO4) =
Ќ2SO4) =  = 0,1326 моль/л.
= 0,1326 моль/л.
“огда мол€рна€ концентраци€ раствора Ќ2SO4 равна:
— (Ќ2SO4) = 0,1326 Ј  = 0,06632 моль/л.
= 0,06632 моль/л.
ƒалее рассчитаем титр “ (Ќ2SO4/Zn), использу€ формулу
“ (Ќ2SO4/Zn) =  =
=  = 0,004335 г/мл.
= 0,004335 г/мл.
“итрование смесей. –аздельное определение смеси кислот или смеси оснований возможно, если константы диссоциации компонентов смеси различаютс€ между собой более чем 104 раз. ѕри кислотно-основном титровании смесей обычно используют два индикатора Ц метиловый оранжевый и фенолфталеин. »зменение окраски каждого из индикаторов соответствует протеканию различных реакций компонентов смеси с титрантом. ѕо результатам титровани€ смеси с двум€ индикаторами определ€ют объемы титранта, пошедшие на взаимодействие с каждым компонентом смеси.
ѕример. ¬ мерную колбу вместимостью 250 мл поместили 10,0 мл смеси хлороводородной и ортофосфорной кислот, содержимое довели до метки. ¬ две колбы дл€ титровани€ поместили по 15,0 мл (V ал) полученного раствора. Ќа титрование первой пробы с метиловым оранжевым израсходовали 27,4 мл (V T1) раствора NaOH с концентрацией 0,09678 моль/л. Ќа нейтрализацию второй пробы в присутствии фенолфталеина затратили 33,2 мл (V T2) раствора NaOH той же концентрации. –ассчитать массу хлороводородной и ортофосфорной кислот в исходной смеси.
–ешение. –ассмотрим кривые титровани€ хлороводородной и ортофосфорной кислот (рис. 4.5). HCl Ц сильна€ кислота, и на ее кривой титровани€ наблюдаетс€ большой скачок (ΔрЌ = 4Ц10). ¬ этом случае можно использовать два индикатора: и метиловый оранжевый (ΔрЌ = 3,1Ц4,4), и фенолфталеин (ΔрЌ = 8,2Ц10,0).

–ис.4.5 ривые титровани€ хлороводородной
и ортофосфорной кислот
Ќа кривой титровани€ H3PO4 наблюдаетс€ два скачка: первый соответствует оттитровыванию H3PO4 до NaH2PO4. ≈му соответствует изменение окраски метилового оранжевого. ¬торой скачок соответствует оттитровыванию H3PO4 до Na2HPO4 и фиксируетс€ по изменению окраски фенолфталеина.
ѕри титровании смеси хлороводородной и ортофосфорной кислот в присутствии метилового оранжевого протекают реакции:
| H—l + NaOH = NaCl + 2H2O H3PO4 + NaOH = NaH2PO4 + H2O | } | V T1 = 27,4 мл, |
а в присутствии фенолфталеина
| H—l + NaOH = NaCl + 2H2O H3PO4 + 2NaOH = Na2HPO4 + 2H2O | } | V T2 = 33,2 мл. |
ѕри титровании с метиловым оранжевым раствор NaOH расходуетс€ на нейтрализацию всей HCl и H3PO4 по одной ступени, а с фенолфталеином Ц на нейтрализацию всей HCl и H3PO4 по двум ступен€м. “огда объем раствора NaOH, пошедшего на титрование ортофосфорной кислоты по одной ступени:
|
|
|
D V = 33,2 Ц 27,4 = 5,8 мл.
ѕо закону эквивалентов определим концентрацию ортофосфорной кислоты в мерной колбе:
— 1 =  =
=  = 0,03742 моль/л.
= 0,03742 моль/л.
онцентраци€ H3PO4 в исходной анализируемой смеси (10,0 мл) равна:
— 2 =  = 0,9355 моль/л.
= 0,9355 моль/л.
ѕо справочнику найдем значение ћr(H3PO4) = 97,9952 г/моль, тогда масса H3PO4 в исходной анализируемой смеси
m (H3PO4) = 0,9355 Ј 10,0 Ј 10Ц3 Ј 97,9952 = 0,9168 г.
ћассу H—l можно рассчитать на основании результатов титровани€ и в присутствии метилового оранжевого, и в присутствии фенолфталеина.
ќбъем раствора NaOH, пошедший на титрование хлороводородной кислоты в присутствии метилового оранжевого
V (NaOH) = 27,4 Ц 5,8 = 21,6 мл.
ќбъем раствора NaOH, пошедший на титрование хлороводородной кислоты в присутствии фенолфталеина
V (NaOH) = 33,2 Ц 2 Ј 5,8 = 21,6 мл,
т. е. численные значени€ в том и другом случае совпадают. «атем рассчитаем концентрацию H—l в мерной колбе:
— 1(HCl) =  = 0,1394 моль/л.
= 0,1394 моль/л.
“огда концентраци€ H—l в исходной смеси (10,0 мл) составл€ет:
— 2 (HCl) =  = 3,4841 моль/л.
= 3,4841 моль/л.
–ассчитаем массу H—l в исходной смеси:
m (HCl) = 3,4841 Ј 10,0 Ј 10Ц3 Ј 36,461 = 1,2703 г.
ќбратное титрование. –езультат обратного титровани€ всегда рассчитываетс€ по разности между количеством молей эквивалента титранта, вз€того на реакцию с определ€емым веществом, и оставшимс€ количеством другого титранта после реакции.
ѕример. Ќавеску технического образца BaCO3 массой 0,3197 г растворили в 20,0 мл 0,2000 ћ раствора Ќ—l. ѕосле полного удалени€ диоксида углерода избыток кислоты оттитровали 8,6 мл 0,09705 ћ раствора ќЌ. Ќайти массовую долю (%) BaCO3 и ¬aO в образце.
–ешение. «апишем уравнени€ протекающих реакций:
BaCO3 + 2 HCl = BaCl2 + CO2 ↑ + H2O
HCl + KOH = KCl + H2O
—ущность обратного титровани€ отражает следующа€ формула:
ν( BaCO3) = ν(HCl) Ц ν( ќЌ)
BaCO3) = ν(HCl) Ц ν( ќЌ)
— учетом условий задачи выразим число молей эквивалентов BaCO3 через количества реагирующих веществ:
ν( BaCO3) = 0,2000 Ј 20,0 Ј 10Ц3 Ц 0,09705 Ј 8,6 Ј 10Ц3 = = 3,165 Ј 10Ц3 моль.
BaCO3) = 0,2000 Ј 20,0 Ј 10Ц3 Ц 0,09705 Ј 8,6 Ј 10Ц3 = = 3,165 Ј 10Ц3 моль.
“огда масса BaCO3 равна:
m (BaCO3) = ν( BaCO3) Ј Mr(
BaCO3) Ј Mr( BaCO3),
BaCO3),
где Mr( BaCO3) Ц мол€рна€ масса эквивалента BaCO3. ѕодставл€€ численные значени€, получим:
BaCO3) Ц мол€рна€ масса эквивалента BaCO3. ѕодставл€€ численные значени€, получим:
m (BaCO3) = 3,165 Ј 10Ц3 Ј 98,67 = 0,3123 г.
–ассчитаем массовую долю BaCO3:
ω(BaCO3) =  = 97,70%.
= 97,70%.
ћассу BaO рассчитываем аналогично, так как ν( BaCO3) = = ν(
BaCO3) = = ν( BaO):
BaO):
m (BaO) = 3,165 Ј 10Ц3 Ј 76,67 = 0,2427 г.
ω(BaO) =  = 75,90%.
= 75,90%.
ѕостроение кривых титровани€ и подбор индикаторов. ѕри построении кривых кислотно-основного титровани€ необходимо провести 4 типа расчетов рЌ раствора в отдельные моменты титровани€:
1) до начала титровани€;
2) до точки эквивалентности (область буферных растворов);
3) в точке эквивалентности;
4) после точки эквивалентности.
¬ыбор индикатора осуществл€ют на основании правила выбора индикатора: дл€ каждого данного титровани€ можно примен€ть только те индикаторы, показатель титровани€ которых лежит в пределах скачка рЌ на кривой титровани€. »нтервал перехода окраски индикатора должен полностью или частично укладыватьс€ на скачок рЌ кривой титровани€. р“ индикатора должен по возможности совпадать со значением рЌ в т. э. или быть близким к этому значению.
|
|
|
ѕример. ѕостроить кривую титровани€ C6H5COOH (бензойной кислоты) (— 0= 0,20 моль/л) раствором ќЌ c концентрацией 0,40 моль/л и подобрать индикаторы.
–ешение. Ѕензойна€ кислота Ц слаба€ кислота (рKа = 4,21), а KќЌ Ц сильное основание, поэтому в данном случае рассмотрим титрование слабой кислоты сильным основанием.
C6H5COOH + KOH = C6H5COOK + H2O
ѕредположим, что дл€ титровани€ вз€ли 100,00 мл (V 0) раствора C6H5COOH (— 0 = 0,20 моль/л). ѕо закону эквивалентов рассчитаем объем раствора ќЌ (Vх), необходимый дл€ полного оттитровывани€ C6H5COOH.
100,00 Ј 0,20 = Vx Ј 0,40; Vx = 50,00 мл.
1. рЌ исходного раствора рассчитаем по формуле дл€ расчета рЌ раствора слабой кислоты:
рЌ =  рKа Ц
рKа Ц  lg — 0 =
lg — 0 =  4,21 Ц
4,21 Ц  lg 0,20 = 2,45.
lg 0,20 = 2,45.
2. ¬ любой момент титровани€ до точки эквивалентности в растворе существует буферна€ смесь, состо€ща€ из неоттитрованной бензойной кислоты и образовавшегос€ бензоата кали€.
ѕри добавлении 50% от эквивалентного количества раствора гидроксида кали€ (25,00 мл) рЌ равен:


ѕри добавлении 90% от эквивалентного количества раствора гидроксида кали€ (45,00 мл) рЌ равен:

≈сли добавлено 99% от эквивалентного количества раствора ќH (49,50 мл), рЌ равен:

ѕри добавлении 99,9% от эквивалентного количества раствора гидроксида кали€ (49,95 мл) рЌ равен:
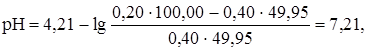
что соответствует точке начала скачка титровани€.
3. ¬ точке эквивалентности вс€ бензойна€ кислота прореагировала и превратилась в соль, концентраци€ которой — соли = — кислоты = 0,2 моль/л (без учета разбавлени€). рЌ раствора соли слабой кислоты рассчитаем по формуле
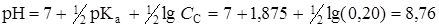 .
.
4. ѕосле точки эквивалентности величина рЌ раствора определ€етс€ только избытком добавленного титранта. ѕри добавлении 50,05 мл раствора ќЌ (100,1 %) рЌ равен:
рЌ = 14 + lg  = 10,30.
= 10,30.
Ёто значение рЌ соответствует точке конца скачка титровани€.
ѕри добавлении 50,50 мл раствора ќЌ (101%)
рЌ = 14 + lg  = 11,30.
= 11,30.
ѕри добавлении 55,00 мл раствора ќЌ (110%)
рЌ = 14 + lg  = 12,30.
= 12,30.
–езультаты сведем в табл. 4.1, по данным которой построим кривую титровани€ (рис. 4.6).
“аблица 4.1 »зменение рЌ в процессе титровани€ —6Ќ5—ќќЌ
| ќбъем титранта V T, мл | рЌ-определ€ющий компонент | ‘ормула дл€ расчета рЌ | рЌ |
| —6Ќ5—ќќЌ | рЌ =  р а Ц р а Ц  lg — 0 lg — 0
| 2,45 | |
| 45,00 | —6Ќ5—ќќЌ —6Ќ5—ќќK | 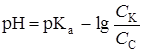
| 5,16 |
| 49,50 | —6Ќ5—ќќЌ —6Ќ5—ќќK | ЂЦї | 6,21 |
| 49,95 | —6Ќ5—ќќЌ —6Ќ5—ќќK | ЂЦї | 7,21 |
| 50,00 | —6Ќ5—ќќK | рЌ = 7 +  р а + р а +  lg — c lg — c
| 8,76 |
| 50,05 | KOH | pH = 14+lg — T | 10,30 |
| 50,50 | KOH | ЂЦї | 11,30 |
| 55,00 | ќЌ | ЂЦї | 12,30 |
—качок титровани€ находитс€ в интервале значений рЌ 7,21Ц10,30. ƒл€ данного титровани€ подход€т индикаторы: нейтральный красный (ΔрЌ = 6,8Ц8,4; р“ = 8,0), фенолфталеин (ΔрЌ = 8,2Ц10,0).

|
| –ис. 4.6 рива€ титровани€ 0,20 моль/л раствора —6Ќ5—ќќЌ 0,40 моль/л раствором KOH |
¬озможно использование фенолового красного (ΔрЌ = 6,8Ц8,4; р“ = 8,0) и тимолового синего (ΔрЌ = 8,0Ц9,6; р“ = 9,0). »нтервалы перехода окраски выбранных индикаторов лежат в пределах скачка кривой титровани€.






