«адание. ƒана окислительно-восстановительна€ реакци€ (номер реакции соответствует варианту).
ќпределите: а) окислитель и восстановитель, б) тип окислительно-восстановительной реакции, в) мол€рные массы эквивалента окислител€ и восстановител€.
»спользу€ метод ионно-электронного баланса расставьте коэффициенты в схемах реакций.
1. HNO2 Ѓ HNO3 + NO + H2O
2. FeS2 + HNO3 Ѓ Fe(NO3) 3 + H2SO4 + NO2 + H2O
3. CuO + NH3 Ѓ Cu + N2 + H2O
4. NaBr + H2SO4 + NaBrO3 = Br2 + Na2SO4 + H2O
5. HClO + H2O2 Ѓ HCl + O2 + H2O
6. Cl2 + KOH Ѓ KCl + KClO3 + H2O
7. S + NaOH Ѓ Na2S + Na2SO3 + Ќ2ќ
8. Al + KClO4 + H2SO4 Ѓ KCl + Al2(SO4)3 + H2O
9. H2S + SO2 Ѓ S + H2O
10. HIO3 + H2O2 Ѓ I2 + O2 + H2O
11. SO2 + NaIO3 + H2O Ѓ I2 + Na2SO4 + H2SO4
12. Sn + HNO3 Ѓ N2O + Sn(NO3)2 + H2O
13. Bi2S3 + HNO3 Ѓ Bi(NO3)3 + NO + S + H2O
14. H3PO3 + H3PO3Ѓ H3PO4 + PH3
15. KNO2 + KMnO4 + H2SO4 Ѓ KNO3 + MnSO4 + K2SO4 + H2O
16. Sb2S3 + HNO3 Ѓ HSbO3 + H2SO4 + NO2 + H2O
17. Na2SO3 + I2 + H2O Ѓ Na2SO4 + HI
18. SnCl2 + NaOH + Bi(NO3)3 Ѓ Bi + Na2SnO3 + H2O
19. As2S3 + HNO3 Ѓ H3AsO4 + H2SO4 + NO + H2O
20. KIO3 + KI + HCl Ѓ KCl + I2 + H2O
21. H3PO3 + KMnO4 + HNO3 Ѓ H3PO4 + KNO3 + Mn(NO3)2 + H2O
22. HMnO4 + NaCl + H2SO4 Ѓ MnSO4 + Cl2 + Na2SO4 + H2O
23. Bi(NO3)3 +K2SnO2 + KOH Ѓ Bi + K2SnO3 + KNO3 + H2O
24. KI + H2O2 +HCl Ѓ I2 + KCl + H2O
25. PH3 + KMnO4 + H2SO4 Ѓ H3PO4 + MnSO4 + K2SO4 + H2O
26. Se + AuCl3 + H2O Ѓ Au + H2SeO3 + HCl
27. Cr2(SO4)3 + Br2 + NaOH Ѓ Na2CrO4 + NaBr + Na2SO4 + H2O
28. Cu(NO3)2 + KI Ѓ CuI + I2 + KNO3
29. Co + HNO3 Ѓ Co(NO3)2 + N2 + H2O
30. Hg + NaNO3 + H2SO4 Ѓ Hg2SO4 + Na2SO4 + NO + H2O
Ёнергетика
» Ќјѕ–ј¬Ћ≈Ќ»≈ ’»ћ»„≈— »’ ѕ–ќ÷≈——ќ¬
’имическа€ термодинамика
“ермодинамика изучает возможность и невозможность самопроизвольного перехода системы из одного состо€ни€ в другое и энергетические эффекты этих переходов. ¬о многих случа€х процессы в термодинамических системах протекают при посто€нном объеме или посто€нном давлении. »з первого закона термодинамики следует, что при этих услови€х теплота Q €вл€етс€ функцией состо€ни€. ѕри посто€нном объеме теплота равна изменению внутренней энергии, а при посто€нном давлении Ц изменению энтальпии Ќ. ≈диница измерени€ энтальпии Ц кƒж/моль. »зменение энтальпии D Ќ равно тепловому эффекту химической реакции, протекающей в изобарно-изотермичес-ких услови€х (р = const, T = const), когда единственным видом работы €вл€етс€ работа расширени€ газа. ≈сли D Ќ < 0, процесс сопровождаетс€ выделением теплоты в окружающую среду (экзотермическа€ реакци€), если D Ќ > 0, процесс идет с поглощением теплоты (эндотермическа€ реакци€).
ƒл€ того чтобы облегчить сравнение энтальпий различных химических реакций, используют пон€тие Ђстандартного состо€ни€ї. —тандартное состо€ние Ц это состо€ние чистого вещества при давлении 1атм и заданной температуре. Ёнтальпию реакции между веществами, наход€щимис€ в стандартных состо€ни€х при температу-
ре T, обозначают 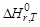 (r означает Ђreactionї). ¬ термохимических уравнени€х указывают не только формулы веществ, но и их агрегатные состо€ни€ или кристаллические модификации.
(r означает Ђreactionї). ¬ термохимических уравнени€х указывают не только формулы веществ, но и их агрегатные состо€ни€ или кристаллические модификации.
|
|
|
“епловой эффект химического процесса, протекающего в услови€х р, “ = const, W = p× D V, не зависит от пути его протекани€, а зависит от природы и физического состо€ни€ исходных веществ и продуктов реакции (закон √есса).
ѕрактическое значение закона √есса состоит в том, что с его помощью можно вычислить неизвестную теплоту реакции путем комбинировани€ термохимических уравнений других изученных реакций.
—ледствие 1 закона √есса. — термохимическими уравнени€ми реакций можно обращатьс€ как с алгебраическими уравнени€ми. ≈сли реакци€ превращени€ вещества 1 в вещество 2: 1 Ѓ 2, характеризуетс€ изменением энтальпии D H 1Ѓ2, а реакци€ 3 Ѓ 2 характеризуетс€ D H 3Ѓ2, то реакци€ 1 Ѓ 3 характеризуетс€ изменением энтальпии:
D H 1Ѓ3 = D H 1Ѓ2 Ц D H 3Ѓ2.
—ледствие 2 закона √есса. “епловой эффект химической реакции равен разности теплот образовани€ продуктов реакции и исходных веществ с учетом стехиометрических коэффициентов:

где j Ц дл€ продуктов реакции; i Ц дл€ исходных веществ; n Ц стехио-метрические коэффициенты,  Ц стандартные теплоты образовани€ веществ.
Ц стандартные теплоты образовани€ веществ.
—тандартной энтальпией (теплотой) образовани€ вещества
(f означает Ђformationї) при заданной температуре называют энтальпию реакции образовани€ одного мол€ этого вещества из элементов, наход€щихс€ в наиболее устойчивом стандартном состо€нии. Ёнтальпи€ образовани€ наиболее устойчивых простых веществ в стандартном состо€нии равна 0 при любой температуре. —тандартные энтальпии образовани€ веществ при температуре 298 приведены в справочниках. Ёнтальпи€ образовани€ соединени€ Ц мера его термодинамической устойчивости.
—ледствие 3 закона √есса. “епловой эффект химической реакции равен разности теплот сгорани€ исходных веществ и продуктов реакции с учетом стехиометрических коэффициентов:
 ,
,
где j Ц дл€ продуктов реакции; i Ц дл€ исходных веществ; n Ц стехио-метрические коэффициенты;  Ц теплоты сгорани€ веществ.
Ц теплоты сгорани€ веществ.
—тандартной энтальпией (теплотой) сгорани€ вещества называют энтальпию реакции полного окислени€ одного мол€ вещества. Ёто следствие обычно используют дл€ расчета тепловых эффектов органических реакций.
¬ технических расчетах используют удельную теплоту сгора-
ни€ QT, котора€ равна количеству теплоты, выдел€ющейс€ при сгорании 1 кг жидкого или твердого вещества и 1 м3 газообразного вещества до образовани€ высших оксидов:
 или
или 
где ћ Ц масса 1 моль вещества; 22,4 л Ц объем 1 моль газа.
≈сли расчет теплоты сгорани€ ведетс€ применительно к реакции с образованием жидкой воды, то удельна€ теплота сгорани€ называетс€ высшей, а дл€ реакции с образованием газообразной воды Ц низшей.
Ёнтропи€ S Ц так же, как и энтальпи€, €вл€етс€ термодинамической функцией состо€ни€ системы:
S = R× ln W,
где R Ц мол€рна€ газова€ посто€нна€, 8,31 ƒж/моль, ; W Ц термодинамическа€ веро€тность состо€ни€ системы.
“ермодинамической веро€тностью состо€ни€ системы называетс€ число микрососто€ний, с помощью которых осуществл€етс€ данное макрососто€ние.
¬еличина S пр€мо пропорциональна ln W, поэтому энтропи€ €вл€етс€ мерой неупор€доченности системы. ≈диница измерени€ энтро-пии Ц ƒж/(моль× ).
≈е значение увеличиваетс€ с ростом температуры и уменьшаетс€ с ее понижением. ѕри повышении давлени€ энтропи€ газа уменьшаетс€, а при понижении Ц увеличиваетс€.
|
|
|
Ќа величину S вли€ют все факторы, св€занные с природой вещества, например, пол€рность однотипных молекул Ц S HCl(г) < S HB r (г) <
< S HI(г),мол€рна€ масса молекул Ц S (F2) < S (Cl2) < S (Br2).
Ёнтропи€ возрастает при переходе вещества из кристаллического состо€ни€ в жидкое и из жидкого в газообразное. ”сложнение молекул также сопровождаетс€ ростом энтропии.
»зменение энтропии D S в процессе химической реакции можно подсчитать, использу€ следствие из закона √есса:
 .
.
—тандартное изменение энтропии в химической реакции при
“ = 298 легко вычислить, использу€ таблицы термодинамических величин, в которых привод€тс€ стандартные энтропии веществ S 0 при “ = 298 .
ѕри химических взаимодействи€х одновременно измен€ютс€ энтальпи€ и энтропи€ системы, откуда возникла иде€ сопоставлени€ энтальпийного D Ќ и энтропийного “ D S факторов химической реакции.
≈сли тенденци€ к пор€дку и беспор€дку в системе одинаковы, то D Ќ = “ D S, что €вл€етс€ математическим условием равновесного состо€ни€ системы, из которого ее можно вывести только путем внешнего воздействи€.
¬ процессе перехода системы из одного состо€ни€ в другое происход€т изменени€ D Ќ ¹ “ D S.
”стойчивость любой системы определ€етс€ соотношением энтальпийного и энтропийного факторов, которые объедин€ютс€ функцией, называемой энергией √иббса D G, котора€ равна
D G = D H Ц T D S, [D G ] = кƒж/моль.
»зменение энергии √иббса учитывает одновременно изменение энтальпии и энтропии системы, суммиру€ тенденции к пор€дку и беспор€дку при переходе системы из одного состо€ни€ в другое. »менно поэтому D G Ц критерий, определ€ющий направление самопроизвольного протекани€ химических процессов.
»з уравнени€ D G = D H Ц T D S следует:
1) если D Ќ <0 и D S >0, то всегда D G < 0, т.е. реакци€, протекающа€ с выделением теплоты и увеличением степени беспор€дка, возможна при всех температурах;
2) если D Ќ >0 и D S <0, то всегда D G >0, т.е. реакци€ с поглощением теплоты увеличением степени пор€дка невозможна ни при каких температурах;
3) во всех остальных случа€х (D Ќ < 0 и D S <0 и если D Ќ >0 и D S > 0, знак D G <0 зависит от соотношени€ членов D Ќ и D S;
4) чем выше температура, тем большее значение приобретает член “ ×D S и при высоких температурах даже эндотермические реакции станов€тс€ самопроизвольными.
—тандартную энергию √иббса реакции рассчитывают по следствию закона √есса:
 ,
,
где D f G 0 Ц стандартные значени€ энергии √иббса.
—тандартные значени€ энергий √иббса образовани€ простых веществ в стандартных состо€ни€х и модификаци€х равны нулю.
»зменение стандартной энергии √иббса при химической реакции может быть вычислено по уравнению:

≈сли пренебречь изменением D Ќ 0и D S 0 с увеличением температуры, то можно определить температуру “ равн, при которой устанавливаетс€ равновесие химической реакции дл€ стандартного состо€ни€ реагентов:

≈сли реагенты наход€тс€ в состо€ни€х, отличных от стандартного, то изменение энергии √иббса рассчитываетс€ по уравнению, по-лучившему название изотермы ¬антЦ√оффа, которое дл€ реакции
ај + b¬ = с— + dD записываетс€ в виде:

где р (ј, ¬, —, D ) Ц относительные парциальные давлени€ соответствующих газов.
”читыва€, что D G 0 = Ц RT× ln K, где Ц константа равновеси€, то изотерма ¬антЦ√оффа будет иметь вид:

ѕримеры решени€ задач
1. »звестны тепловые эффекты следующих реакций (1) и (2) при 273 и посто€нном давлении 101,3 кѕа. –ассчитать при тех же услови€х тепловой эффект реакции (3).
(1) 
 =
= 
(2)  ½
½ 


(3) 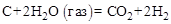
 =?
=?
–ешение. ƒл€ определение теплового эффекта реакции воспользуемс€ следствием 1 закона √есса. “ермохимическое уравнение реак-ции (3) можно получить в результате следующей комбинации термохимических уравнений реакций (1) и (2): (3) = (1) Ц 2 × (2)

|
|
|
 .
.
—ледовательно:  ;
;
 кƒж.
кƒж.
2. ¬ычислите тепловой эффект образовани€ NH3 из простых веществ при стандартном состо€нии по тепловым эффектам реакций:
(1) 2Ќ2 + ќ2 = 2Ќ2ќ(ж), D Ќ 01 = Ц571,68 кƒж,
(2) 4NH3 + 3O2 = 6Ќ2ќ(ж) + 2N2, D Ќ 02 = Ц1530,28 кƒж.
–ешение. «апишем уравнение реакции, тепловой эффект которой необходимо определить:
(3) ½ N2 + 3/2 Ќ2 = NH3, D f H 0(NH3) =?
¬ уравнени€ (1) и (2) вход€т Ќ2O(ж) и ќ2, которые не вход€т в уравнение (3). ѕоэтому, чтобы исключить их из уравнений (1) и (2), умножим уравнение (1) на 3 и вычтем из него уравнение (2):
6Ќ2 + 3ќ2 Ц 4NH3 Ц 3ќ2 = 6Ќ2ќ(ж) Ц 6Ќ2ќ(ж) Ц 2N2(г). (4)
ѕосле преобразовани€ уравнени€ (4) и делени€ его на 4 получаем искомое уравнение (3).
јналогичные действи€ проделаем с тепловыми эффектами:
(D Ќ 01×3 Ц D Ќ 02):4 = D Ќ 03.
¬ результате получаем: [Ц571,68×3 Ц (Ц1530,28)]:4 = Ц46,19 кƒж,
т.е. D f H 0(NH3) = Ц46,19 кƒж/моль.
3. ¬ычислите теплоту гидратации —аCl2, если известно, что при растворении 1 моль безводного —а—l2 выдел€етс€ 72,7 кƒж, а при растворении 1 моль кристаллогидрата CaCl2×6Ќ2ќ поглощаетс€ 18,0 кƒж теплоты.
–ешение. ѕроцесс растворени€ в воде хлорида кальци€ можно разбить на две стадии:
(1) CaCl2(к) + 6Ќ2ќ(ж) = —а—l2×6Ќ2ќ(к); D Ќ 01
(2) —а—l2×6Ќ2ќ(к) + aq = —а—l2 aq (ж) + 6Ќ2ќ(ж); D Ќ 02.
ѕерва€ стади€ Ц процесс гидратации, т.е. получение кристаллогидрата, тепловой эффект которой нужно рассчитать; втора€ стади€ Ц растворение кристаллогидрата в воде. —уммарный тепловой эффект D Ќ 02 + D Ќ 01 равен теплоте растворени€ безводной соли DЌ03:
—а—l2(к) + aq = —aCl2 aq (ж); D Ќ 03.
–азность теплот растворени€ безводной соли (DЌ03) и растворени€ кристаллогидрата (D Ќ 02) представл€ет собой теплоту гидратации (D Ќ 01).
ѕодставив соответствующие значени€ тепловых эффектов, получаем:
D Ќ 0гидр = Ц72,7 Ц (+18,0) = Ц90,7 кƒж,
т.е. при гидратации 1 моль CaCl2 выдел€етс€ 90,7 кƒж теплоты.
4. акое из перечисленных соединений HF(г), HCl(г) и HBr(г), наход€щихс€ в стандартном состо€нии, €вл€етс€ наиболее устойчивым, т.е. будет разлагатьс€ при более высоких температурах.
–ешение. “епловые эффекты реакций, протекающих в пр€мом и обратном направлени€х, равны по величине и противоположны по знаку. Ёто означает, что если известны стандартные теплоты образовани€ данных соединений, то энтальпии разложени€ этих соединений будут равны, но противоположны по знаку энтальпии образовани€. „ем прочнее молекула, тем больше энергии необходимо затратить на ее разложение.
D f H 0(HF)= Ц270,7; D f H 0(HCl)= Ц92,30; D f H 0(HBr)= Ц35,98(кƒж/моль).
»з трех соединений наиболее устойчивым €вл€етс€ HF, так как на разложение 1 моль этого соединени€ потребуетс€ 270,7 кƒж теплоты, т.е.
D f Ќ 0HF(г) = ЦD Ќ 0разлож.ЌF(г).
5. ќпределить тепловой эффект реакции в стандартных услови€х (“ = 298 ).
 .
.
–ешение. ƒл€ определение теплового эффекта реакции воспользуемс€ с ледствием 2 закона √есса. —тандартные теплоты образовани€ исходных веществ и продукта реакции находим в справочнике:
¬ещество  , кƒж/моль
, кƒж/моль
 Ц1675,0
Ц1675,0
 Ц395,2
Ц395,2
 Ц3434,0
Ц3434,0
ѕоскольку в справочнике  приводитс€ в расчете на 1 моль, то при расчете теплового эффекта реакции соответствующее значение
приводитс€ в расчете на 1 моль, то при расчете теплового эффекта реакции соответствующее значение  умножаетс€ на число моль вещества, участвующего в реакции.
умножаетс€ на число моль вещества, участвующего в реакции.

 Ц3434,0Ц ( Ц1675,0) Ц 3 (Ц395,2) = Ц573,4 (кƒж/моль).
Ц3434,0Ц ( Ц1675,0) Ц 3 (Ц395,2) = Ц573,4 (кƒж/моль).
ƒанна€ реакци€ идет с поглощением теплоты ( > 0), т.е. €вл€етс€ эндотермической.
> 0), т.е. €вл€етс€ эндотермической.
6. ѕо известным значени€м тепловых эффектов реакций сгорани€ алмаза и графита рассчитайте тепловой эффект превращени€ одного мол€ углерода в форме алмаза в графит ( =?).
=?).
–ешение. ќпределим энтальпии реакций сгорани€ графита и алмаза:
|
|
|
(1) —графит + ќ2 = —ќ2; D Ќ 01 = Ц396,3 кƒж/моль,
(2) —алмаз + ќ2 = —ќ2; D Ќ 02 = Ц399,197 кƒж/моль,
D Ќ 02=  +(
+( +
+  ) = Ц 396,3 Ц 1,897 Ц 0 =
) = Ц 396,3 Ц 1,897 Ц 0 =
= Ц 398,197 кƒж/моль.
„тобы из уравнений (1) и (2) получить уравнение перехода алмаза в графит с неизвестным тепловым эффектом, достаточно из второго вычесть первое:
— алмаз = — графит, D Ќ 03 =?
и соответственно,
D Ќ 03 = D Ќ 02 Ц D Ќ 01 = Ц398,197 Ц (Ц396,3) = Ц1,897 кƒж/моль.
7. —колько теплоты выделитс€ при сжигании 20 литров этилена, вз€того при нормальных услови€х, если известны стандартные теплоты образовани€ веществ.
–ешение. «апишем уравнение процесса горени€ этилена:
—2Ќ4(г) + 3ќ2 = 2—ќ2(г) + 2Ќ2ќ(ж)

ѕодставив справочные данные, получим:
D Ќ хим.реак = 2×(Ц396,3) + 2×(Ц285,84) Ц 52,28 = Ц1130,72 кƒж/моль.
—ледовательно, при сжигании 1 моль —2Ќ4 выдел€етс€ 1130,72 кƒж.
ќднако по условию задачи сжигаетс€ 20 л этилена, что составл€ет
n моль =  моль,
моль,
где 22,4 л/моль Ц мольный объем любого газа при н.у. “аким образом, при сгорании 0,89 моль этилена выделитс€:
0,89 моль×(Ц1130,72 кƒж/моль) = Ц1009,57 кƒж теплоты.
8. ѕредскажите знак изменени€ энтропии в следующих реакци€х:
а) N2(г) + 3H2(г) = 2NH3(г),
б) —(к) + Ќ2ќ(г) = —ќ2(г) + Ќ2(г),
в) 2—ќ(г) + ќ2(г) = 2—ќ2(г).
–ешение. »звестно, что энтропи€ газов всегда значительно больше энтропии твердых тел и жидкостей, поэтому в химических реакци€х, идущих с участием газообразных веществ, энтропи€ реакции всегда положительна (D S 0 > 0), если в результате процесса возрастает число молей газообразных веществ и отрицательна (D S 0 < 0), если число молей газообразных уменьшаетс€.
Ќетрудно увидеть, что в реакции (а) число молей газообразных веществ в системе уменьшаетс€ от 4 до 2, поэтому (D S 0(а) < 0); в реакции (б) число молей газообразных веществ возрастает D S 0(б) > 0; в реакции (в) уменьшаетс€ D S 0(в) < 0.
9. ќпределите энтропию реакции
H2 S (г) + Cl2(г) = 2HCl(г) + S (к).
–ешение. ѕоскольку энтропи€ Ц функци€ состо€ни€ системы, то ее изменение D S 0хим.реак в процессе химической реакции можно подсчитать, пользу€сь следствием из закона √есса:

ѕодставив соответствующие значени€ энтропии дл€ каждого из веществ, вз€тые из справочных данных, получаем:
 ƒж/ .
ƒж/ .
10. –ассчитайте энтропийный и энтальпийный факторы протекани€ процесса при стандартных услови€х и 298 :
—ќ2(г) + 4Ќ2(г) = —Ќ4(г) + 2Ќ2ќ(ж).
акой из факторов способствует самопроизвольному протеканию реакции в пр€мом направлении?
–ешение. ƒл€ расчета энтальпийного и энтропийного факторов воспользуемс€ следствием из закона √есса:



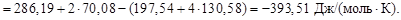
Ёнтальпийным фактором процесса €вл€етс€ энтальпи€ реакции. —амопроизвольному протеканию процесса способствует уменьшение энтальпии системы (D Ќ 0хим.реак). Ёнтропийный фактор равен произ-ведению абсолютной температуры на энтропию реакции, т.е.
“ × D S 0хим.реак. —амопроизвольному течению процесса способствует рост энтропии системы D S 0 > 0.
¬ нашем случае энтропийный фактор равен:
“× D S 0хим.реак = Ц393,51×298 = Ц117265,98 ƒж/моль,
или Ц117,266 кƒж/моль, величина которого не способствует самопроизвольному протеканию процесса.
Ёнтальпийный фактор D Ќ хим.реак = Ц253 кƒж способствует самопроизвольному протеканию процесса в пр€мом направлении.
11. –ассчитайте стандартную энергию √иббса химической реакции при 298 и установите возможность ее самопроизвольного протекани€ в пр€мом направлении.
CuSO4(к) + 2NH4OH(ж) = Cu(OH)2(ж) + (NH4)2SO4(к).
–ешение. Ёнерги€ √иббса D G Ц термодинамическа€ функци€ состо€ни€ системы. »зменение энергии √иббса химической реакции может быть рассчитано на основании следстви€ из закона √есса:


Ћюба€ химическа€ реакци€ протекает самопроизвольно в том направлении, которое отвечает при заданных услови€х (давлении и температуре) уменьшению величины G.
¬ данном случае D G 0хим.реак < 0, следовательно, возможно само-произвольное протекание процесса в пр€мом направлении.
12. ”становите, возможно ли при температурах 298 и 1000 образование вод€ного газа по уравнению: —(графит) + Ќ2ќ(г) = Ќ2(г) +
+—ќ(г),полага€, что все газы наход€тс€ при давлении 1 атм.
ƒл€ данной реакции определите температуру начала самопроизвольного процесса при стандартных состо€ни€х веществ.
–ешение. ¬ справочных таблицах найдем значени€ D f Ќ 0и S 0298дл€ исходных веществ и продуктов реакции.
| ¬ещество | D f Ќ 0, кƒж/моль | S 0298, ƒж/(моль× ) |
| —(т) | 5,74 | |
| Ќ2ќ(г) | Ц241,98 | 188,9 |
| Ќ2(г) | 130,7 | |
| —ќ(г) | Ц110,6 | 197,7 |
–ассчитаем стандартные энтальпию и энтропию реакции:




Ёнерги€ √иббса химической реакции равна
|
|
|
D G 0хим.реак = D Ќ 0хим.реак Ц “× D S 0хим.реак .
–ассчитаем D G 0хим.реак при 298 :
D G 0хим.реак = 131,38 Ц 298 ×0,134 = 91,48 кƒж.
ѕоскольку D G 0хим.реак > 0,то реакци€ синтеза вод€ного газа при 298 самопроизвольно не идет.
ѕрин€в изменение энтальпии и энтропии реакции посто€нными в температурном интервале 298Ц1000 можно рассчитать изменение энергии √иббса при 1000 : D G 0хим.реак = 131,38Ц1000×0,134 = Ц2,62 кƒж,
т.е. реакци€ взаимодействи€ графита с вод€ным паром при 1000 становитс€ самопроизвольной. »з реакции синтеза вод€ного газа видно, что если энтропи€ системы увеличиваетс€, то с ростом температуры веро€тность реакции тоже увеличиваетс€.
ƒл€ определени€ температуры, выше которой произойдет смена знака энергии √иббса (при стандартных услови€х), воспользуемс€ уравнением:

следовательно, при T > 980Kи стандартном состо€нии реагентов реакци€ может протекать самопроизвольно в пр€мом направлении.
13. ”становите, возможно ли восстановление оксида железа (III) до свободного металла по уравнению:
Fe2O3(к) + 3H2(г) = 2Fe(к) + 3Ќ2ќ(г)
при температуре 298 и при начальных парциальных давлени€х веществ р (Ќ2) = 1,5; р (Ќ2ќ) = 0,9.
–ешение. »зменение энергии √иббса химической реакции рассчитаем на основании следстви€ из закона √есса:


»звестно, что изменение энергии √иббса D G хим.реак при любых начальных парциальных давлени€х веществ св€зано с D G 0хим.реак уравнением, получившим название изотермы ¬антЦ√оффа, которое дл€ данной реакции запишетс€ следующим образом:

отсюда

так как D G хим.реак > 0, то процесс невозможен.
14. ¬ычислить константу равновеси€ дл€ реакции синтеза аммиака из стандартных энергий образовани€ √иббса веществ.
–ешение. «апишем уравнение реакции синтеза аммиака из простых веществ:
N2(г) + 3H2(г) = 2NH3(г).
—тандартное изменение энергии √иббса дл€ указанной реакции составл€ет:


онстанта равновеси€ дл€ “ = 298 при D G хим.реак = 0находитс€ из соотношени€:

откуда







