1. Загальні поняття теорії розчинів електролітів
Електролітами називаються сполуки, які у розчинах чи розплавах розпадаються (дисоціюють) на йони і які при накладенні різниці потенціалів проводять електричний струм. Електроліти є провідниками другого роду, тому що електричний струм утворюється внаслідок руху іонів (на відміну від провідників першого роду – металів, в яких струм зумовлюється рухом електронів).
Розчини електролітів характеризуються ступенем дисоціації - a, який є співвідношенням кількості молекул, які розпались на іони до загальної кількості молекул:
a = n дис / n заг.
В залежності від чисельного значення a електроліти розділяють на сильні та слабкі. До сильних електролітів (a > 30 %) відносять майже всі солі, з кислот – сульфатну, нітратну, хлоридну, хлорну; з основ – розчинні основи металів першої та другої групи NaOH, KOH, Ca(OH)2, Ba(OH)2.
До слабких електролітів (a < 3 %) належать з кислот: сульфідна, нітритна, карбонатна, сульфурводнева, більшість одноосновних органічних кислот (ацетатна, бензойна, тощо); з основ – розчин амоніаку в воді – амоній гідроксид, всі нерозчинені основи р -, d -, і f -елементів (Сu(OH)2, Fe(OH)3, Al(OH)3, тощо). Фосфорна кислота є кислотою середньої сили.
За величиною a розподілити електроліти на сильні і слабки можна тільки приблизно, тому що ця характеристика залежить від природи розчинника, температури та концентрації розчину.
Процес електролітичної дисоціації слабких електролітів зручніше характеризувати константою хімічної рівноваги, яку в цьому випадку називають константою дисоціації. Так для реакції KА «К+ + А–, відповідно до закону діючих мас, константа дисоціації K д дорівнює:
K рівн = [ K+] [A–] / [ KA] = K дис.
Величина K залежіть від природи електроліта, розчинника та температури і не залежіть від концентрації розчину. Чим більше K дис, тим більше дисоціює електроліт. Наприклад, K дис(СН3СООН) = 1 . 10–5, K дис(Н2СО3) = 4,5 . 10–7, тобто ацетатна кислота, більш сильна, ніж карбонатна.
Багатоосновні кислоти та багатокислотні основи дисоціюють ступінчасто і кожна з ступенів має свою константу К дис. Наприклад:
Н3РО4 «Н+ + Н2РО4- K 1дис =7,5.10–3;
Н2РО4- «Н+ + НРО42- K 2дис = 6,3.10–8;
НРО42- «Н+ + РО43-- K 3дис = 1,3.10–12.
Сумарна константа знаходиться за рівнянням K дис = K 1 K 2 K 3.
При ступінчастій дисоціації речовин подальший ступень дисоціації характеризується меншим розпадом, ніж попередній, тобто K 1 > K 2 > K 3. Це пояснюється тим, що енергія, яку треба затратити для відривання йона, мінімальна в разі його відривання від нейтральної молекули і стає більшою при дисоціації у кожному наступному процесі.
2. Використання законів ідеальних розчинів до розбавлених розчинів електролітів
У своїх дослідженнях Вант-Гофф довів, що розчини електролітів підкоряються законам ідеальних розчинів тільки при введені поправочного коефіцієнту, якій він назвав ізотонічним коефіцієнтом (і). Ізотонічний коефіцієнт зумовлений зростанням кількості частинок у розчині при дисоціації. Для цих розчинів математичні вирази законів ідеальних розчинів мають вигляд:
D Р = іР Ао c В; D Т зам = іК кр Сm; D Т кіп = іК еб С m; Р осм = іСмRT.
Ці закономірності діють тільки для розбавлених розчинів. Коефіцієнт і змінюється від одиниці до числа, яке дорівнює кількості йонів, на які розпадається електроліт. При подальшому розбавленні розчину і зростає. Наприклад: і (0,2 М розчина КСl) = 1,81, і (0,02 М розчину КСl) = 1,96. Між і та a існує зв язок: a = і -1/ n -1, де n – кількість іонів, на які дисоціює електроліт.
Приклад 1. Визначити ізотонічний коефіцієнт для розчину, який містить 8 г NaOH у 1000 г Н2О, що закіпає при 100,184 °С. К еб(Н2О) = 0,516 °С.
Розвʼязання:
1. і –?
Другий закон Рауля для розчинів електролітів виражається рівнянням D t кіп = іК еб С m; Сm = ni . (1000/ m роз-ка) =  . 1000/ m роз-ка, звідки
. 1000/ m роз-ка, звідки
і = (D t кі . m роз-ка . M (NaOH): (К еб . 1000 . m (NaOH) = (0,184×100×40):(0,516×1000×8) = =1,78.
Відповідь: і = 1,78.
3 .Теорія слабких електролітів
Якщо концентрацію слабкого електроліту, що розкладається на два іони
АВ «А+ +В–
позначити через С, а ступінь його дисоціації у розчині через a, то концентрація кожного з іонів буде a С, а концентрація недисоційованих молекул С (1– a).Тоді рівняння константи дисоціації матиме вигляд:
K дис = С2a2/С(1– a)
Це рівняння є законом розбавлення Оствальда. Він дає можливість визначити ступінь дисоціації при різних концентраціях, але не виражає залежність константи дисоціації від ступеня дисоціації, тому що K дис є стала величина для конкретного електроліту. Для розчинів, в яких a досить мала (a ®0) рівняння набуває вигляду K дис = a2 С або α =  K дис є сталою, табличною величиною, С = СМ, a зростає при розбавленні розчину. Це відбувається внаслідок зменшення кількості потрібних зіткнень іонів.
K дис є сталою, табличною величиною, С = СМ, a зростає при розбавленні розчину. Це відбувається внаслідок зменшення кількості потрібних зіткнень іонів.
Приклад 2. Розрахувати ступінь дисоціації ацетатної кислоти у 0,1 М та 0,001 М розчинах. K дис(СН3СООН) = 10−5.
Розвʼязання:
Використовуємо закон розбавлення Оствальда:
α(0,1 М) = 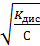 =
=  = 10−2 = 0,01.
= 10−2 = 0,01.
α(0,001 М) = 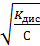 =
=  = 10−1 = 0,1.
= 10−1 = 0,1.
Відповідь: α(0,1 М р-ну СН3СООН) = 0,01, α(0,001 М р-ну СН3СООН) = 0,01.
4 .Теорія сильних електролітів. Активність
Теорія сильних електролітів була розроблена Дебаєм та Гюккелем у 1923 році. Згідно з нею відмінності у поведінці сильних та слабких електролітів пов¢язані з міжіонними взаємодіями в розчинах сильних електролітів.
Сильними електролітами є сполуки, які мають кристалічну гратку координа-ційного типа з великою ступеню іонності. Прикладом таких сполук є більшість солей.Для характеристики цих розчинів не використовуються поняття ступені і константі дисоціації, тому що вони враховують присутність деякої кількості недисоційованих молекул.
За певних умов (наприклад, при великих концентраціях) іони протилежних знаків можуть наближуватись один до одного і утворювати іонну пару (асоціат), тобто асоціювати.
Не слід отожнювати поняття недисоційованої молекули і йонної пари. У йонних парах відстань між іонами більше, ніж у молекулах.
В розбавлених розчинах електроліт іонізований повністю. У більш-менш концентрованих розчинах електроліти утворюють іонні асоціати під дією міжіонної взаємодії. Внаслідок цього, експериментальні дані, які залежать від кількості частин у розчині (D Р, D Т кип, D Т плав, тощо) виявляються меншими, ніж розраховані значення.
Для кількісних розрахунків Л¢юіс запропонував використовувати формальне уявлення про активну концентрацію або активність (а) замість концентрації і замість ступені дисоціації -уявну (позирну) ступінь дисоціації (aуяв). Активність - це допоміжна розрахункова термодинамічна функція, яка в сумарній формі характеризує ступінь зв¢язанності молекул компонента. Активність пов¢язана з істиною концентрацією співвідношенням:
a = fC,
де f - коефіцієнт активності. Активність вимірюється в тих же одиницях, що і концентрація, а f - безрозмірна величина. Для дуже розбавлених розчинів a = C. Якщо у закони Рауля підставити значення a замість С, ці рівняння будуть справедливі і для розчинів електролітів.
Коефіцієнт активності є функцією не тільки концентрації розчину. Його значення залежіть також від природи електроліту, температури та йонної сили розчину.
Іонна сила розчину m(І) є півсумою добутку концентрації усіх іонів в розчині на квадрат їхнього заряду z.
m = 1/2(С 1 z 12 + C 2 z 22 + … + C n z n2)
Вважається, що при сталій m коефіцієнти активності теж сталі, залежать тільки від m і не залежать від концентрації.
Прямих методів визначення f немає, тому їх значення обчислюють за відповідними емпіричними формулами. Наприклад, для розраховуючі f для бінарних електролітів з С £ 0,01 моль/л використовують формулу lg f = -0,5 z + . z - Öm.
Деякі величини f для поширених іонів в залежності від їх заряду і іонної сили розчину надані у таблицях.
Введення поняття про активність дозволяє, не виявляючи складної картини взаємодії у розчинах сильних електролітів, оцінити сумарний ефект цієї взаємодії, ступень її відхилення від ідеальних розчинів і використати закони ідеальних розчинів для аналізу реальних систем.
5. Іонно - молекулярні реакції
При нейтралізації будь-якої сильної кислоти будь-якою сильною основою на кожний моль води, що при цьому утворюється, виділяється близько 57,6 кДж теплоти:
НСІ +NаОН = NаСІ + Н2О; D Н = -57,53 кДж
НNО3 + КОН = КNО3 + Н2О; D Н = -57,61 кДж
Це свідчить про те, що всі подібні реакції зводяться до одного процесу - процесу утворення води:
Н+ + ОН– ® Н2О.
Взагалі реакція утворення води з іонів оборотна, але вода – дуже слабкий електроліт, тому рівновага цієї реакції зміщена у бік утворення молекул і реакція нейтралізації відбувається до кінця.
Розглянемо ще один приклад:
AgNO3 + HCl ® AgCl¯ + HNO3;
Ag2SO4 + CuCl2 ® 2AgCl¯ + CuSO4.
Обидва ці процеси також зводяться до процесу утворення осаду аргентум хлориду:
Ag+ + Cl– ® AgCl¯.
У наведених прикладах процеси зводились до утворення або малодисоцийованої речовини, або до утворення осаду. Взагалі, будь-які реакції між іонами відбуваються тоді, коли внаслідок їх взаємодії утворюється малорозчинна, летка сполука, слабкий електроліт, або комплексна сполука.
2НСІ + К2СО3 ® КСІ + СО2 + Н2О;
2Н+ + СО32– ® СО2 + Н2О;
Ni(NO3)2 + 6NH3 ® [Ni(NH3)6](NO3)2;
Ni 2+ + 6NH3 ® [Ni(NH3)6]2+.
Приклад 3. Записати рівняння утворення осаду купрум (ІІ) гідроксиду при взаємодії купрум сульфату з натрій гідроксидом у йонному та молекулярному виді.
Розв¢язання:
Молекулярне рівняння: CuSO4 + NaOH ® Cu(OH)2 + Na2SO4;
повне йонне рівняння: Cu+2 + SO42– + Na+ + 2OH– ® Cu(OH)2 ¯+ 2Na+ + SO42–;
коротке йонне рівняння: Cu+2 + 2OH– ® Cu(OH)2 ¯.
6. Теорії кислот і основ
1. Теорія електролітичної досоціації С. Ареніуса (1887) була першою обгрунтованою теорією розчинів. Згідно з нею, кислотами є речовини, у водних розчинах яких присутні іони Н+, що виникають при дисоціації кислоти. Всі загальні властивості кислот – кислий смак, дія на метали, індикатори, тощо, по суті є властивостями іонів водню. Основами є речовини, у водних розчинах яких присутні іони ОН–. Кислотно-основна взаємодія обовʼязково відбувається за реакцією нейтралізації Н+ + ОН– ®Н2О. Ця теорія повністю підтвердилась для речовин, які реагують у водних розчинах.
Вивчення процесів, які відбуваються без участі розчинника (води), або у неводних розчинниках (наприклад, рідкий амоніак) дозволили створити ряд нових сучасних теорій кислот і основ.
2. Протонна теорія кислот і основ (Бренстед і Лоурі, 1923). Протон – це унікальна частина. Він приблизно у 10000 разів менше інших іонів, має виключну рухливість, здатність глибоко проникати у електронну оболонку інших атомів і йонів, утворювати водневі зв’язки. Ці обставини сприяли появленню протонної теорії.
Згідно з нею, кислотою є будь-яка частина (молекула або іон) яка віддає протон. Основою є будь-яка частина, яка приєднує протон. Кислоти і основи можуть бути трьох типів:
1. нейтральні молекули – типові кислоти НСІ, H2SO4, т.і. та типові основи NaOH, KOH;
2. аніонні кислоти та основи:
HSO4– «H+ + SO42–, HPO42– «H+ + PO43–;
Cl– + H+ «HCl, OH– + H+ «H2O.
3. катіонні кислоти та основи:
NH4+ «NH3 + H+, H3O+ «H2O + H+;
NH3 + H+ «NH4+, H2O + H+ «H3O+.
Будь-яка реакція відщеплення протона, згідно з цією теорією, виражається схемою:
кислота «основа + Н+.
Кислота і основа, які беруть участь у даному процесі називаються супряженими. Наприклад, H3O+, HSO4– - кислоти, їм супряжені основи H2O та SO42–. Протонна теорія пояснює основний характер органічних сполук - амінів, етерів, кетонів та ін.
В цій теорії розчинники розділяються на протолітичні і апротонні. Протолітічні розчинники в результаті власної дисоціації утворюють протони:
Н2О «Н+ + ОН–;
СН3СООН «СН3СОО– + Н+.
Апротонні розчинники – це інертні розчинники – бензен, толуен, дихлоретан. Вони не утворюють протони.
Сила кислот і основ залежіть від природи сполуки, тобто від взаємного впливу атомів і груп атомів в молекулі. Сила кислот залежіть також від розчинника. Наприклад, у воді НСІ – сильна кислота, у бензені – дуже слабка. Це пояснюється тим, що вода йонізує молекули хлороводню і сприяє дисоціації.
Сила кислот і основ характеризується їх константою дисоціації (іноді використовують логарифм константи з протилежним знаком). Наприклад,
K ис(СН3СООН) = 10–5, KА = -lg(10–5) = 5;
K дис(NH4OH) = 10–5, KВ = 5.
Важливе значення в протонній теорії має власна дисоціація розчинника, причому йон Гідрогену, якій утворюється миттєво проникає у електронні оболонки молекул розчинника утворюючи йони ліонію (по аналогії з гідроксонієм).
7. Дисоціація води
Вода є слабким електролітом, a = 1,8 10–9. Однією з причин такої незначної дисоціаці є те, що цій процес пригнічується дією водневих зв¢язків.
Дисоціацію води можна записати у вигляді рівнянь:
Н2О «Н+ + ОН– (1)
Реакції цього типу звуться реакціями автоіонізації, або автопротолізу. В них одна молекула води грає роль кислоти (відщеплює протон), друга - роль основи (приєднує протон). Гідратований протон Н3О+ називається іоном гідроксонію. Утворення при дисоціації води катіонів Н3О+ і аніонів ОН– свідчить про амфотерну природу води.
Для спрощення і зручності запису найчастіше використовують рівняння (1). Для цього рівняння К дис = [H+] [OH–] / [H2O].
СМ (Н2О) = 1000:18 = 55,56 моль/л; [H+] = [OH–] = CM × a = 55,56·1,8×10–9 = 10–7 моль/л.
В цьому рівнянні a дуже мала, [H2O] = const;
К дис [H2O] = [Н+]]OH–] = 10–14 = Kw - іонний добуток води.
Кw є сталою величиною і залежить тільки від температури.
Розчини, у яких концентрація йонів водню і гідроксид-іонів однакові називаються нейтральними розчинами. У кислих розчинах концентрація йонів водню більше, ніж гідроксид-іонів, у лужних - навпаки. Проте, якими б не були ці концентрації, їх добуток лишається сталою величиною. Це дає змогу обчислювати одну з концентрацій, якщо друга відома:
[H+] = 10–14 / [OH–]; [OH–] = 10–14 / [H+].
8.Водневий показник
Записувати концентрації йонів Н+ або ОН– через відʼємний ступінь не зовсім зручно. Тому у 1909 році датський біохімік С.Серенсен запропонував характеризувати їх величиною водневого показника рН, який визначається за формулою
рН = − lg [H+].
Оскільки концентрація йонів водню може змінюватися у межах іонного добутку, то рН змінюється в межах від нуля до чотирнадцяти. У нейтральному розчині рН = 7. У кислому рН < 7, у лужному рН > 7.
Розчини, значення рН яких перебувають в інтервалі від 0 до 3 належать до сильнокислих, від 4 до 6 – до слабкокислих; слабколужні розчини мають рН 8…10, сильнолужні - рН = 11...14.
Для вимірювання рН існують різні методи. Приблизно реакцію розчину можна визначити за допомогою спеціальних реактивів, які називаються індикаторами, або просоченими ними папірцями (метилоранж, метилрот, фенолфталеїн, лакмус, універсальні лакмусові паперці). Більш точніше рН вимірюють пристроями - рН-метрами.
Приклад 4. Визначити рН розчину, якщо концентрація йонів водню у розчині дорівнює 4·10−3 моль/л.
Розв’язання:
рН = −lg[H+] = −lg(4·10−3) = −lg4· − lg10−3 = −0,6 + 3 = 2,4.
Відповідь: рН розчину дорівнює 2,4.
Приклад 5. Визначити концентрацію гідроксид-іонів у розчині з рН = 10,8.
Розв’язання:
1. рОН –?
рН + рОН = 14; рОН = 14 − 10,80 = 3,2.
2. концентрація гідроксид-іонів –?
рОН = −lg[ОH−]; −lg[ОH−] = 3,2;
За таблицею логарифмів знаходимо, що [ОH−] = 6,31·10−4 моль/л.
Відповідь: концентрація гідроксид-іонів дорівнює 6,31·10−4 моль/л.
Приклад 6. Визначити рН 0,17 н. розчину СН3СООН, константа дисоціації якої дорівнює 1,75·10−5.
Розв’язання:
1. α −?
Для слабких електролітів α = 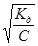 ; α =
; α = 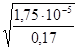 =
=  = 10−2.
= 10−2.
2. [Н+] −?
СН3СООН ↔ СН3СОО− + Н+;
[Н+] = α× СМ (СН3СООН); СМ (СН3СООН) = С н(СН3СООН) = 0,17 М.
[H+] = 0,17·10−2 = 0,17·10−2 моль/л.
3. рН −?
рН = −lg[H+] = −lg 0,17·10−2 = 2,77.
Відповідь: рН розчину дорівнює 2,77.
Приклад 7. Визначити рН 0,01 М розчину гідроксиду амонію, якщо K д = 1,77·10−5.
Розвʼязання:
1. a(NH4OH) −?
NH4OH ↔ NH4+ + OH−; [OH−] = СМ a n, де СМ − молярна концентрація електроліту,
a – ступінь дисоціації, n – кількість іонів даного виду, отриманих при дисоціації (у даному випадку n =1).
a = 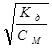 , a =
, a =  =
= 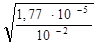 = 0,042.
= 0,042.
2. [OH−] −?
[OH−] =10−2·0,042·1 = 0,42 ·10−3 моль/л,
3. pH −?
pOH = −lg [OH−] = 3,7; pH + pOH = 14; pH = 14 – 3,7 = 10,3.
Відповідь: рН = 10,3
Добуток розчинності






